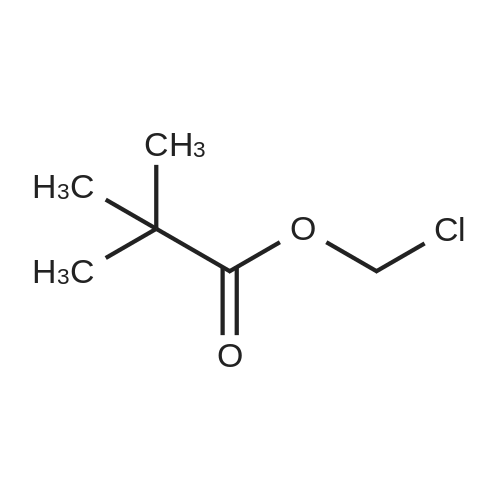
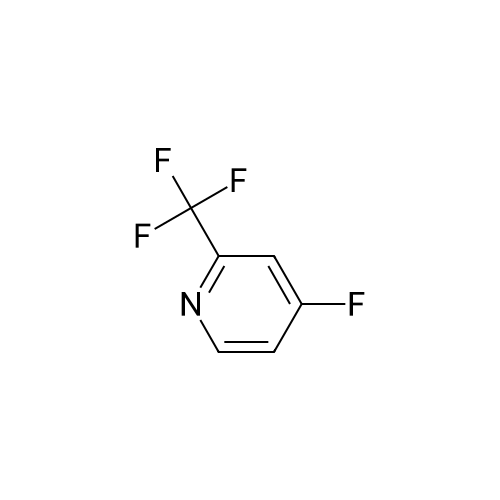
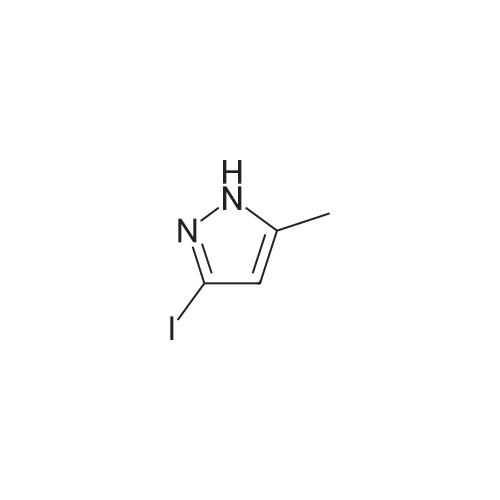
|
With potassium tert-butylate; In N,N-dimethyl-formamide; at 100℃; for 1h; |
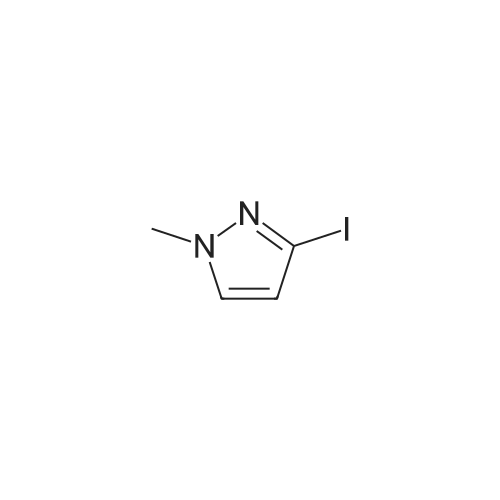
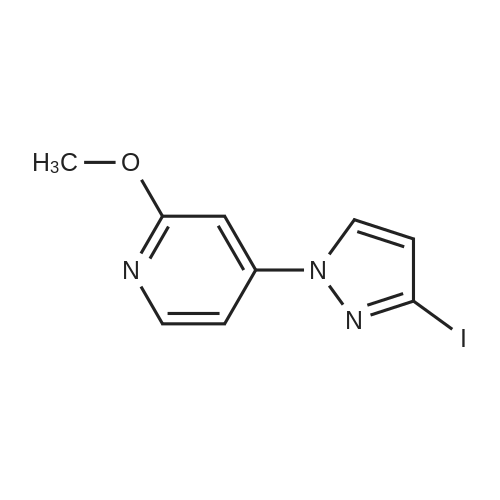
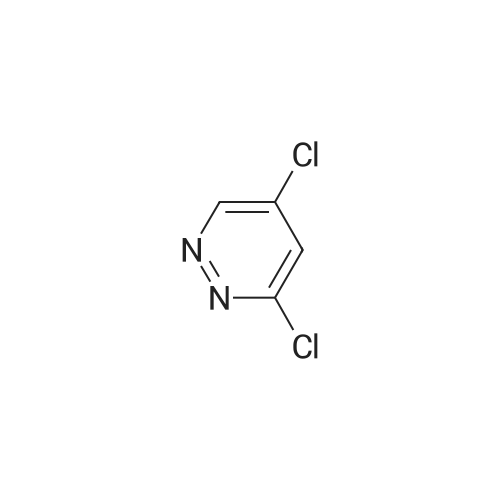
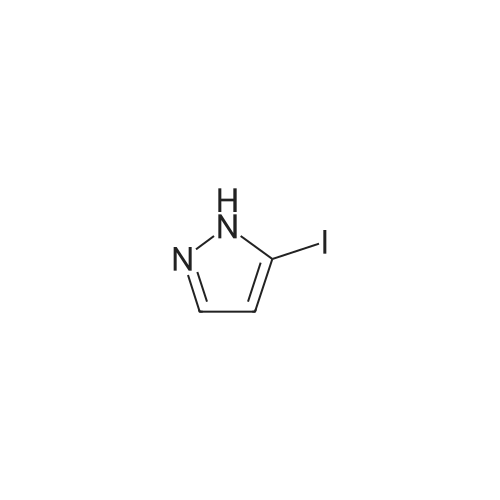
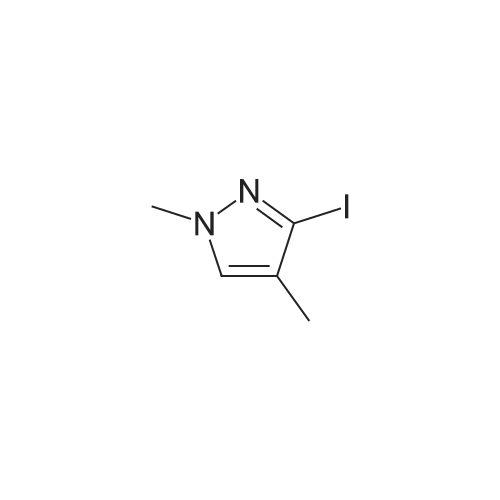
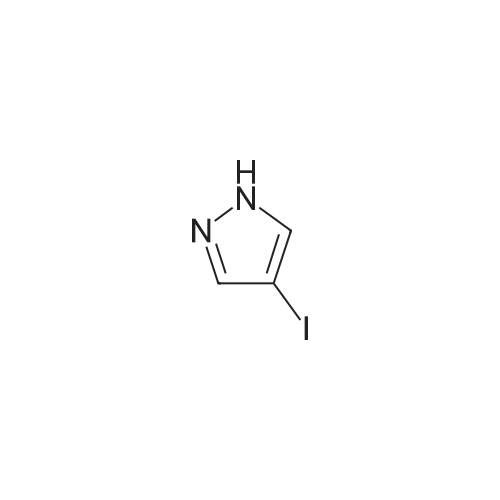
To a soluton of<strong>[4522-35-4]3-iodopyrazole</strong> (500 mg, 2.58mmol) and 3,5-dichloropyridazine(384 mg, 2.58 mmol) in anhydrous DMF (5 mL) at room temperature was addedpotassium tert-butoxide (289 mg, 2.58 mmol) in one portion. It was heated at100 C for 1 h. It was cooled to room temperature, diluted with EtOAc (50 mL),washed with satd aq. NaHC03 (10 mL) and water (100 mL). The aqueous layerwas separated and extracted with EtOAc (3 x 50 mL). The combined organiclayers were washed with water (1 00 mL), brine (1 00 mL), dried over Na2S04,filtered and concentrated. The residue was purified by flash chromatography(ISCO Combiflash, Gold 40 g, 0-60% EtOAc in hexanes) to give 3-chloro-5-(3-iodo-1H-pyrazol-1-yl)pyridazine, as a white solid. LCMS calc.= 306.92, found= 306.96 (M+Ht. 1H NMR (500 MHz, CHCh-d): o 9.54 (d, J= 2.3 Hz, 1 H);7.94 (d, J= 2.7 Hz, 1 H); 7.90 (d, J= 2.3 Hz, 1 H); 6.81 (d, J= 2.7 Hz, 1 H). |
|
With potassium tert-butylate; In N,N-dimethyl-formamide; at 100℃; for 1h; |
3-Chloro-5-(3-iodo-lH-pyrazol-l -vDpyridazine To a soluton of <strong>[4522-35-4]3-iodopyrazole</strong> (500 mg, 2.58 mmol) and 3,5-dichloropyridazine (384 mg, 2.58 mmol) in anhydrous DMF (5 mL) at room temperature was added potassium teri-butoxide (289 mg, 2.58 mmol) in one portion. It was heated at 100 C for 1 h. It was cooled to room temperature, diluted with EtOAc (50 mL), washed with satd aq. NaHC03 (10 mL) and water (100 mL). The aqueous layer was separated and extracted with EtOAc (3 x 50 mL). The combined organic layers were washed with water (100 mL), brine (100 mL), dried over Na2S04, filtered and concentrated. The residue was purified by flash chromatography (ISCO Combiflash, Gold 40 g, 0-60% EtOAc in hexanes) to give 3-chloro-5-(3- iodo-lH-pyrazol-l-yl)pyridazine, as a white solid. LCMS calc. = 306.92, found = 306.96 (M+H)+. NMR (500 MHz, CHCl3-d): delta 9.54 (d, J= 2.3 Hz, 1 H); 7.94 (d, J= 2.7 Hz, 1 H); 7.90 (d, J= 2.3 Hz, 1 H); 6.81 (d, J= 2.7 Hz, 1 H). |
|
With potassium tert-butylate; In N,N-dimethyl-formamide; at 100℃; for 1h; |
INTERMEDIATE 40 3-Chloro-5-(3-iodo-lH-pyrazol-l-yl pyridazine To a soluton of <strong>[4522-35-4]3-iodopyrazole</strong> (500 mg, 2.58 mmol) and 3,5-dichloropyridazine (384 mg, 2.58 mmol) in anhydrous DMF (5 mL) at room temperature was added potassium tert-butoxide (289 mg, 2.58 mmol) in one portion. It was heated at 100 C for 1 h. It was cooled to room temperature, diluted with EtOAc (50 mL), washed with satd aq. NaHC03 (10 mL) and water (100 mL). The aqueous layer was seperated and extracted with EtOAc (3 x 50 mL). The combined organic layers were washed with water (100 mL), brine (100 mL), dried over Na2S04, filtered and concentrated. The residue was purified by flash chromatography (ISCO Combiflash, Gold 40 g, 0-60% EtOAc in hexanes) to give 3-chloro-5-(3-iodo-lH-pyrazol-l-yl)pyridazine, as a white solid. LCMS calc. = 306.92, found = 306.96 (M+H)+. NMR (500 MHz, CHC13- d): 5 9.54 (d, J= 2.3 Hz, 1 H); 7.94 (d, J= 2.7 Hz, 1 H); 7.90 (d, J= 2.3 Hz, 1 H); 6.81 (d, J= 2.7 Hz, 1 H). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping