| 93% |
With air; In benzonitrile; at 200℃; for 24.0h; |
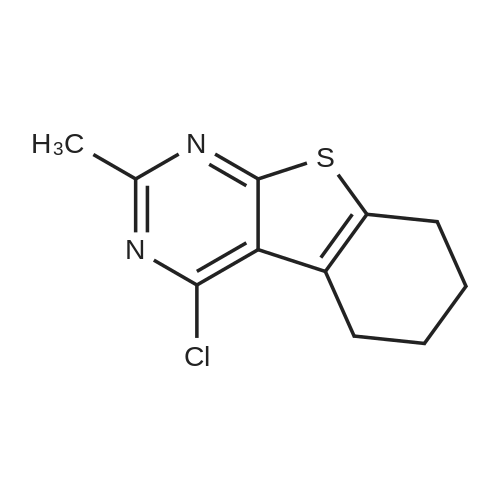
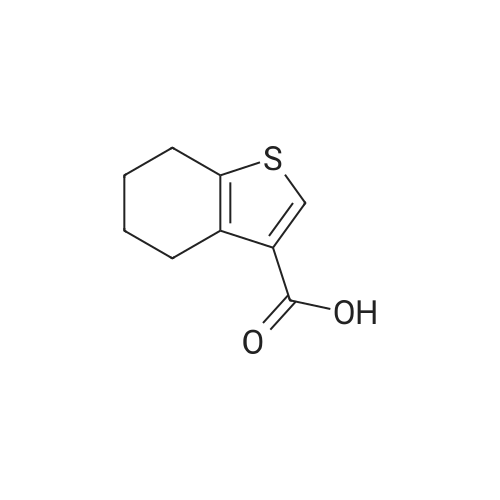
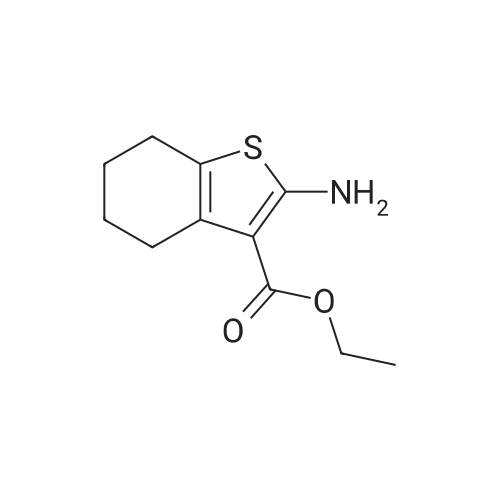
General procedure: A mixture of cyclohexanone (0.196 g,2.0 mmol), methyl cyanoacetate (0.198 g, 2.0 mmol),elemental sulfur (0.064 g, 2.0 mmol) and morpholine (0.174g, 2.0 mmol) was heated at 75 C for 1 h. After nearlycomplete conversion to the corresponding 2-aminothiophene, as was indicated by TLC monitoring, thereaction mixture was cooled to r.t. and the solid residue wasrecrystallized from EtOH to afford methyl 2-amino-4,5,6,7-tetrahydrobenzo[b]thiophene-3-carboxylate (6a). Then asolution of 6a (0.211 g, 1 mmol) in benzonitrile (2 mL) washeated within a flask equipped with an air-filled balloon at200 C for 24 h in a silicone oil bath. Progress of the reactionwas monitored by TLC. After completion of the reaction, the mixture was cooled to r.t. and the excess of benzonitrile wasremoved under the reduced pressure. The crude product waspurified by column chromatography using n-hexane-EtOAc(8:1) as eluent. The solvent was evaporated under thereduced pressure and the residue was crystallized from nhexane-EtOAc (5:1) to afford the pure product 7a as paleyellow crystals. Yield: 0.188 g, 91% (on the basis of 6a |
| 93% |
With benzonitrile; at 200℃; for 24.0h; |
General procedure: A mixture of cyclohexanone (0.196 g,2.0 mmol), methyl cyanoacetate (0.198 g, 2.0 mmol),elemental sulfur (0.064 g, 2.0 mmol) and morpholine (0.174g, 2.0 mmol) was heated at 75 C for 1 h. After nearlycomplete conversion to the corresponding 2-aminothiophene, as was indicated by TLC monitoring, thereaction mixture was cooled to r.t. and the solid residue was recrystallized from EtOH to afford methyl 2-amino-4,5,6,7-tetrahydrobenzo[b]thiophene-3-carboxylate (6a). Then asolution of 6a (0.211 g, 1 mmol) in benzonitrile (2 mL) was heated within a flask equipped with an air-filled balloon at 200 C for 24 h in a silicone oil bath. Progress of the reaction was monitored by TLC. After completion of the reaction, the mixture was cooled to r.t. and the excess of benzonitrile was removed under the reduced pressure. The crude product was purified by column chromatography using n-hexane-EtOAc(8:1) as eluent. The solvent was evaporated under the reduced pressure and the residue was crystallized from nhexane-EtOAc (5:1) to afford the pure product 7a as paleyellow crystals. |
| 41% |
With palladium 10% on activated carbon; In toluene; for 120.0h;Reflux; |
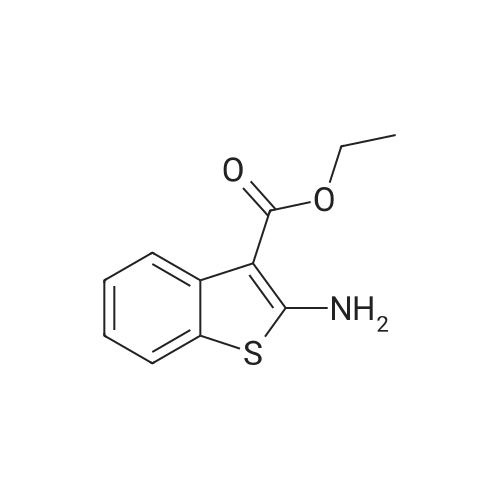
A mixture of 5a (1.8 g, 7.9 mmol) and 10% Pd/C (1.8 g) in toluene (130 mL) was stirred under refluxfor 5 days. The reaction mixture was then cooled to r.t. and filtered over celite. The filtrate was concentrated at reduced pressure to afford a yellow solid residue, which was purified was by flash column chromatography (n-hexane-EtOAc 100:0 v/v increasing to n-hexane-EtOAc 90:10 v/v) to give pure a white solid in 41% yield. TLC (9:1 n-hexane-EtOAc, Rf: 0.28). 1H-NMR (CDCl3), δ: 1.49 (t, J= 7.1 Hz,3H), 4.44 (q, J= 7.1 Hz, 2H), 6.54 (bs, 2H), 7.14-7.17 (m, 1H), 7.32-7.35 (m, 1H), 7.51-7.53 (m, 1H),8.12-8.14 (m, 1H). 13C-NMR (CDCl3), δ: 14.5, 59.8, 99.8, 121.2, 122.3, 122.5, 125.4, 128.8, 137.4,164.3, 166.3. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping