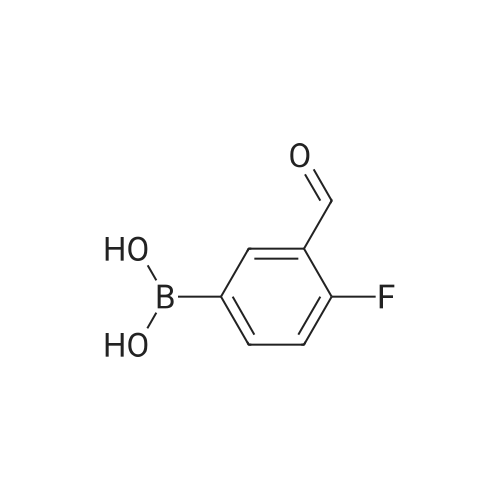
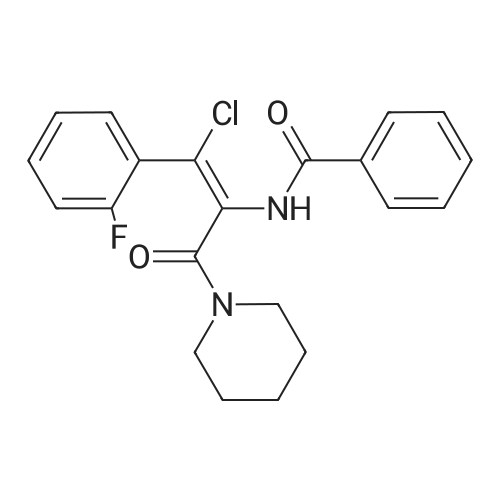
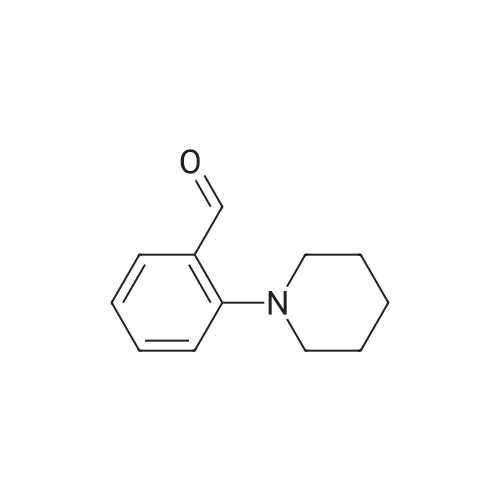
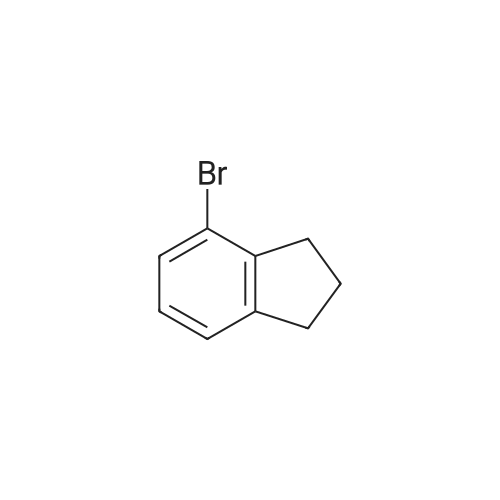
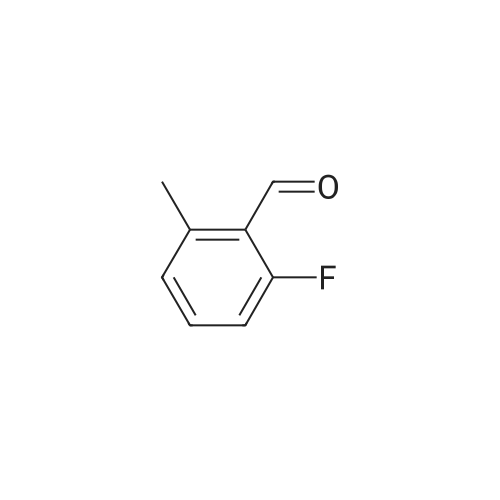
| 79% |
With potassium carbonate; In N,N-dimethyl-formamide; at 150℃; for 1.5h;Schlenk technique; Inert atmosphere; |
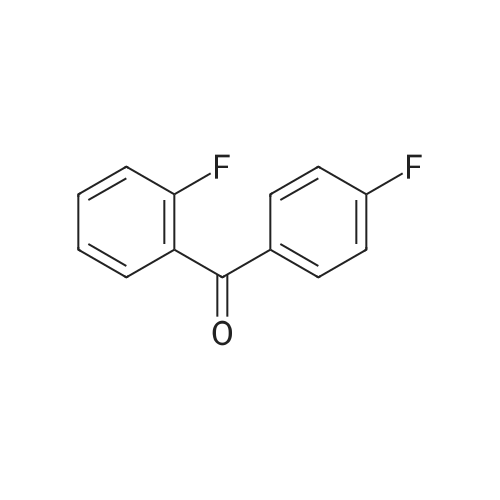
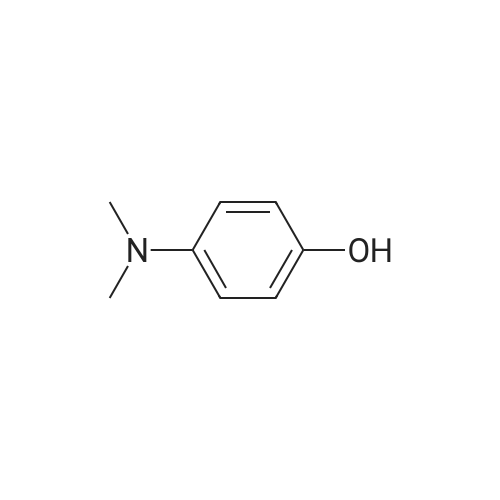
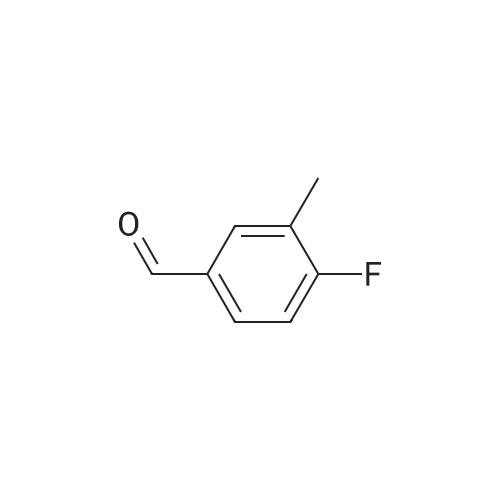
The benzaldehyde intermediates are synthesized following literature procedures with modifications. Into a dry Schlenk flask under argon atmosphere the corresponding phenol (17.7 mmol), 2-fluorobenzaldehyde (2.0 g, 16.1 mmol), potas- sium carbonate (5.6 g, 40.3 mmol) and anhydrous DMF (40 ml_) are added at room temperature. The mixture is warmed in a sealed flask to 170°C and stirred at this temperature for 2 h (for preparing 2-phenoxy-benzaldehyde and 2-(4- chlorophenoxy)benzaldehyde) or at 150°C for 1 .5 h (for preparing 2-(4- (dimethylamino)phenoxy)benzaldehyde). Then the mixture is allowed to cool to room temperature and is treated with water (200 ml_) and the product is extracted with diethyl ether (3^50 ml_). The combined organic layers are washed with NaOH (1 M, 50 ml_), brine (150 ml_), dried over anhydrous MgSO4 and evaporated in vacuum. The residue is purified by column chromatography (cyclohex- ane/ethyl acetate 10:1 , v/v (for preparing 2-phenoxy-benzaldehyde and 2-(4- chlorophenoxy)benzaldehyde) or used in next reaction without purification (in case of 2-(4-(dimethylamino)phenoxy)-benzaldehyde). |
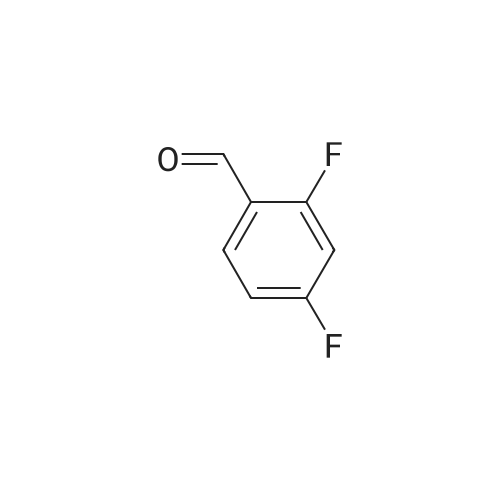
| 79% |
With potassium carbonate; In N,N-dimethyl-formamide; at 150℃; for 1.5h;Schlenk technique; Inert atmosphere; Sealed tube; |
The benzaldehyde intermediates are synthesized following literature procedures with modifications. Into a dry Schlenk flask under argon atmosphere the corresponding phenol (17.7 mmol), 2-fluorobenzaldehyde (2.0 g, 16.1 mmol), potassium carbonate (5.6 g, 40.3 mmol) and anhydrous DMF (40 mL) are added at room temperature. The mixture is warmed in a sealed flask to 170°C and stirred at this temperature for 2 h (for preparing 2-phenoxy-benzaldehyde and 2-(4-chlorophenoxy)benzaldehyde) or at 150°C for 1.5 h (for preparing 2-(4-(dimethylamino)phenoxy)benzaldehyde). Then the mixture is allowed to cool to room temperature and is treated with water (200 mL) and the product is extracted with diethyl ether (3×50 mL). The combined organic layers are washed with NaOH (1 M, 50 mL), brine (150 mL), dried over anhydrous MgS04 and evaporated in vacuum. The residue is purified by column chromatography (cyclohexane/ethyl acetate 10:1, v/v (for preparing 2-phenoxy-benzaldehyde and 2-(4-chlorophenoxy)benzaldehyde) or used in next reaction without purification (in case of 2-(4-(dimethylamino)phenoxy)-benzaldehyde). [0076] 2-(4-(dimethylamino)phenoxy)benzaldehyde is obtained as a white solid (3.07 g, 79percent yield). 1H NMR (300 MHz, CDCl3) delta 10.59 (d, J = 0.8 Hz, 1 H), 7.90 (dd, J = 7.8, 1.8 Hz, 1 H), 7.44 (ddd, J = 8.5, 7.3, 1.8 Hz, 1 H), 7.12 - 7.05 (m, 1 H), 7.03 - 6.97 (m, 2H), 6.84 - 6.74 (m, 3H), 2.96 (s, 6H). [0077] 13C NMR (75 MHz, CDCl3) delta 189.85, 161.75, 148.14, 145.47, 135.76, 128.33, 126.05, 122.23, 121.25, 116.90, 114.18, 41.34. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science













 HazMat Fee +
HazMat Fee +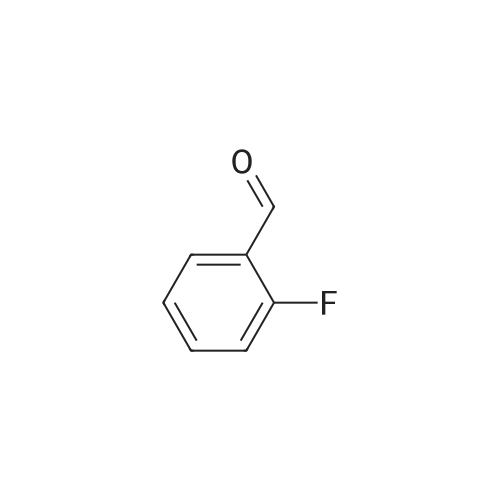

 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping


 Studies of 1, 2-Diarylethylamines as N-Methyl-D-Aspartate Receptor Antagonists.png)