| 21% |
|
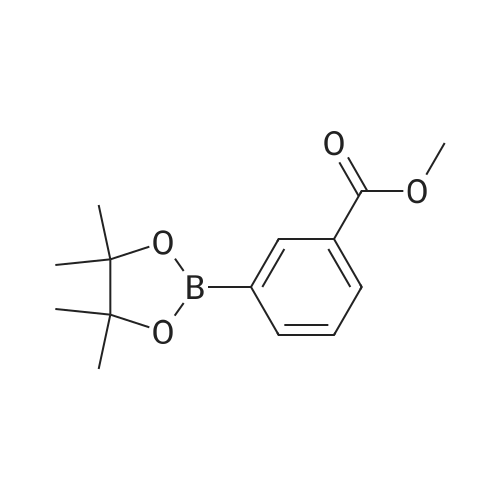
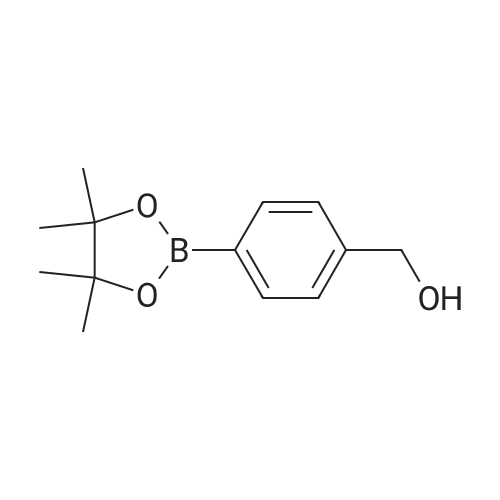
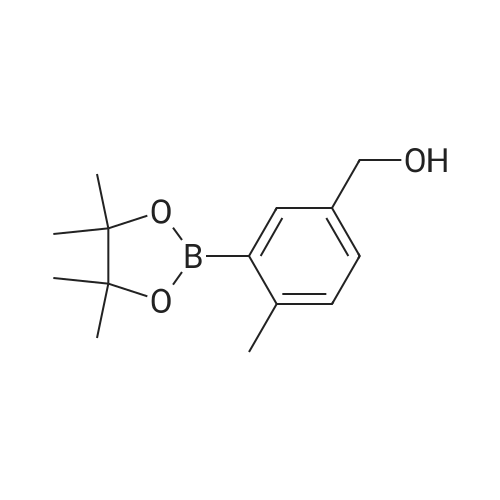
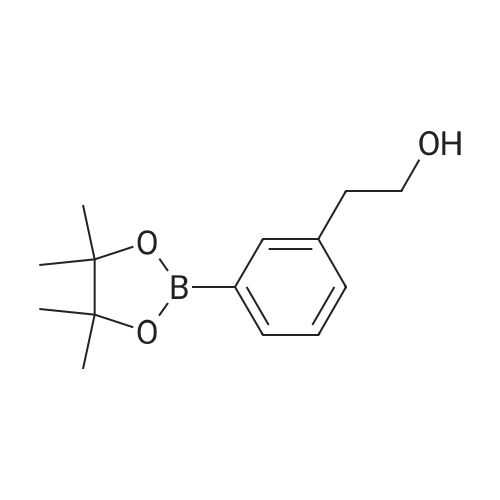
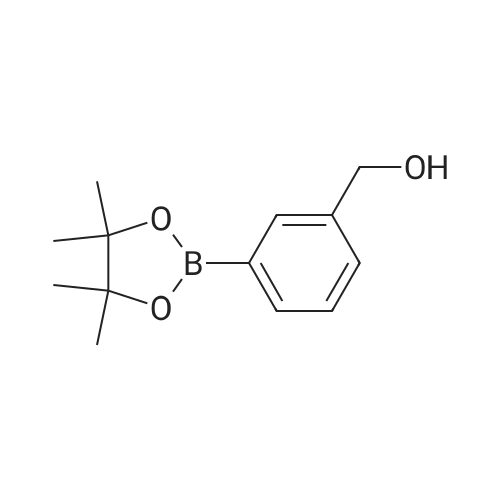
Step 2(S)-(3-(8-(6-(2-Methylpyrrolidin-1-yl)pyridin-2-ylamino)-[1,2,4]triazolo[1,5-a]pyridin-6-yl)phenyl)methanol Procedure:A mixture of (S)-6-chloro-N-(6-(2-methylpyrrolidin-1-yl)pyridin-2-yl)-[1,2,4]triazolo[1,5-a]pyridin-8-amine (100 mg, 0.3 mmol), <strong>[443776-76-9](3-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)phenyl)methanol</strong> (84 mg, 0.36 mmol), Pd2(dba)3 (30 mg, 0.05 mmol), X-Phos (30 mg, 0.06 mmol) and Cs2CO3 (196 mg, 0.6 mmol) in dioxane/H2O (30 mL/5 mL) was stirred at reflux for 18 h under N2 atmosphere. The solvent was removed under reduced pressure and the residue purified by preparative-HPLC (Gemini 5u C18 150×21.2 mm; inject volume: 3 ml/inj, flow rate: 20 mL/min; wavelength: 214 nm and 254 nm; gradient conditions: 50% acetonitrile/50% water (0.1% TFA, v/v) initially, and proceed to 82% acetonitrile/18% water (0.1% TFA, v/v) in a linear fashion after 9 min.) to give (S)-(3-(8-(6-(2-methylpyrrolidin-1-yl)pyridin-2-ylamino)-[1,2,4]triazolo[1,5-a]pyridin-6-yl)phenyl)methanol 222-trifluoroacetate (25 mg, 21%) as a yellow solid. 1H NMR (300 MHz, CD3OD): delta 9.18 (s, 1H), 8.42 (s, 1H), 8.34 (s, 1H), 7.67 (s, 1H), 7.60-7.56 (m, 1H), 7.49-7.35 (m, 3H), 6.23 (d, 1H, J=7.8 Hz), 5.98 (d, 1H, J=8.1 Hz), 4.70 (s, 2H), 4.24-4.20 (m, 1H), 3.59-3.55 (m, 1H), 3.41-3.38 (m, 1H), 2.13-1.98 (m, 3H), 1.72-1.70 (m, 1H), 1.13 (d, 3H, J=6.3 Hz). LC/MS: 401 [M+H]+. HPLC: 100% at 214 nm, 100% at 254 nm, tR=5.54 min. |
| 21% |
|
A mixture of (S)-6-chloro-N-(6-(2-methylpyrrolidin- l-yl)pyridin-2-yl)-[l,2,4]triazolo[l,5-a]pyridin-8-amine (100 mg, 0.3 mmol), (3-(4,4,5,5-tetramethyl- 1,3,2- dioxaborolan-2-yl)phenyl)methanol (84 mg, 0.36 mmol), Pd2(dba)3 (30 mg, 0.05 mmol), X-Phos (30 mg, 0.06 mmol) and Cs2C03 (196 mg, 0.6 mmol) in dioxane/H20 (30 mL/5 mL) was stirred at reflux for 18 h under N2 atmosphere. The solvent was removed under reduced pressure and the residue purified by preparative-HPLC (Gemini 5u C18 150x21.2 mm; inject volume: 3ml/inj, flow rate: 20 mL/min; wavelength: 214 nm and 254 nm;gradient conditions: 50% acetonitrile/50% water (0.1% TFA, v/v) initially, and proceed to 82% acetonitrile/18% water (0.1% TFA, v/v) in a linear fashion after 9 min.) to give (S)- (3-(8-(6-(2-methylpyrrolidin- l-yl)pyridin-2-ylamino)-[l,2,4]triazolo[l,5-a]pyridin-6- yl)phenyl)methanol 222-trifluoroacetate (25 mg, 21 %) as a yellow solid. 1H NMR (300 MHz, CD3OD): delta 9.18 (s, 1H), 8.42 (s, 1H), 8.34 (s, 1H), 7.67 (s, 1H), 7.60 - 7.56 (m, 1H), 7.49 - 7.35 (m, 3H), 6.23 (d, 1H, J = 7.8 Hz), 5.98 (d, 1H, J = 8.1 Hz), 4.70 (s, 2H), 4.24 - 4.20 (m, 1H), 3.59 - 3.55 (m, 1H), 3.41 - 3.38 (m, 1H), 2.13 - 1.98 (m, 3H), 1.72 - 1.70 (m, 1H), 1.13 (d, 3H, J = 6.3 Hz). LC/MS: 401 [M + H]+. HPLC: 100 % at 214 nm, 100 % at 254 nm, tR = 5.54 min. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping