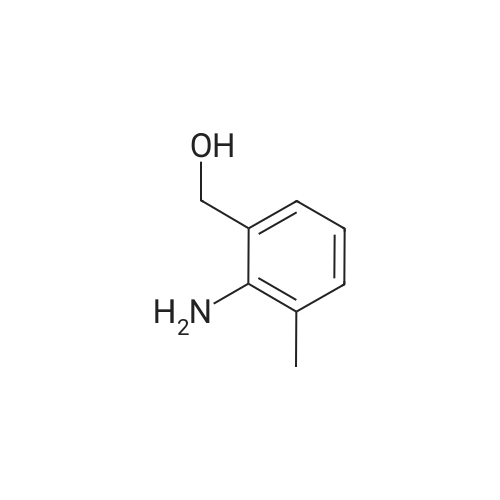
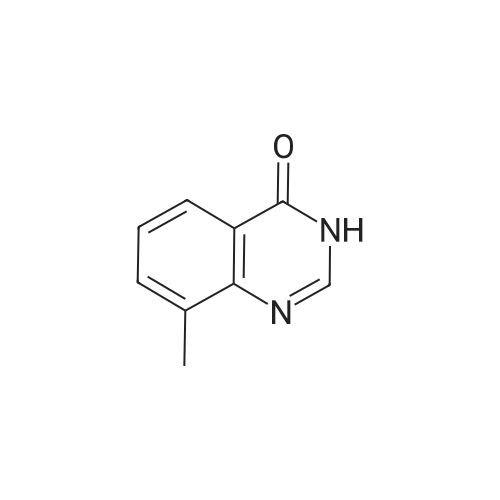
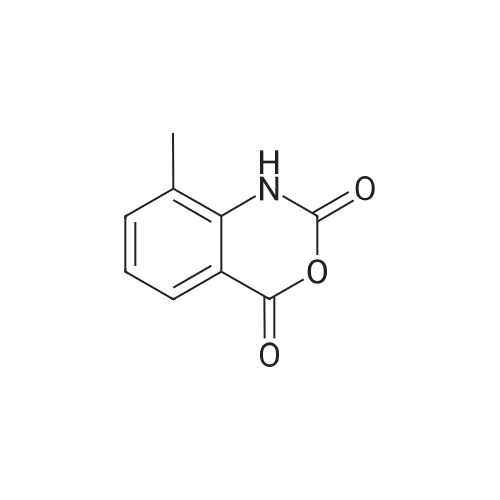
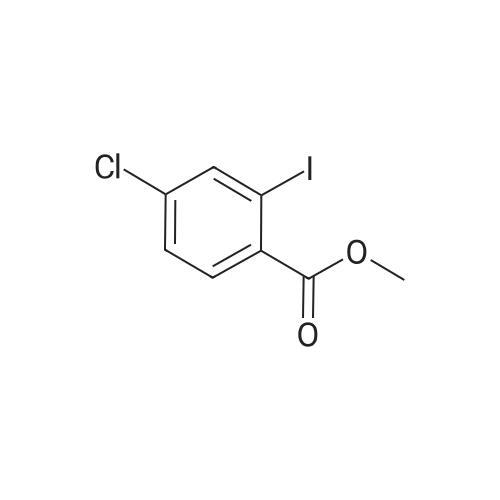
| 98% |
|
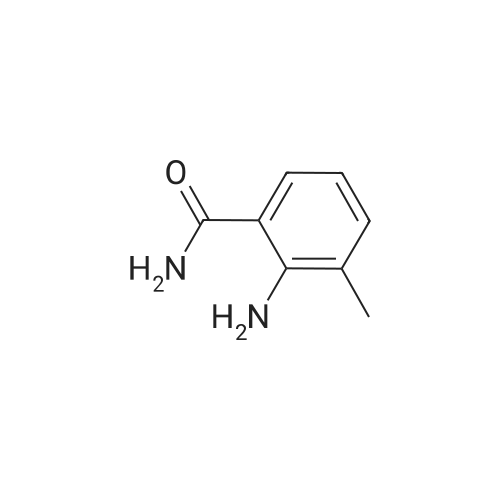
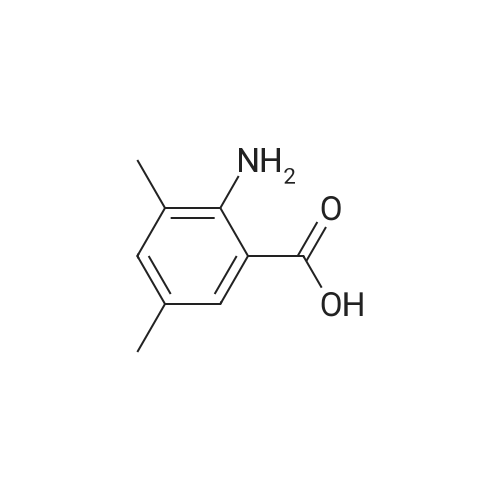
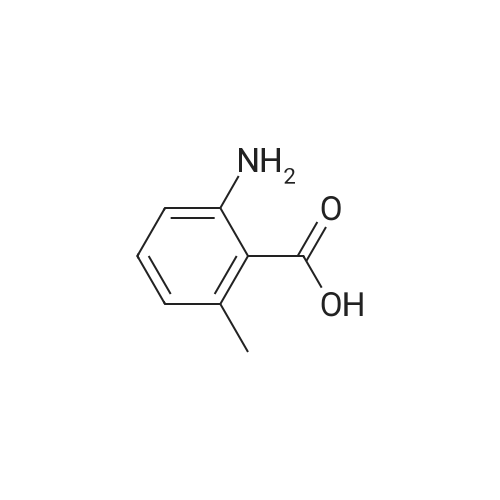
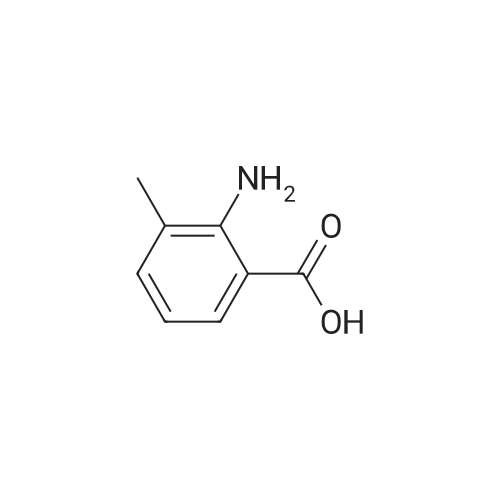
2-Amino-3-methylbenzoic acid (2.93 g, 19.8 mmol) in dry DMF (78 mL) was treated with 1,1-carbonyl-diimidazole (3.14 g, 19.4 mmol) at 70C under Ar for lh, after which aq. NH3 (35%, 49 mL) was added dropwise and the mixture was stirred for 16 h. The mixture was allowed to cool to 20C and was diluted with EtOAc (100 mL). The mixture was washed with water (2 x 40 mL) and brine (2 x 40 mL). The organic solution was dried and the solvent was evaporated to give (8) (2.14 g, 98%) as a white solid: mp 150-152C (lit.24 mp 150-152C); NMR ((CD3)2SO) delta 2.05 (3 H, s,), 6.35 (2 H, br), 6.41 (1 H, brt, / = 7.6 Hz,), 7.00 (1 H, brs), 7.04 (1 H, d, / = 6.8 Hz), 7.34 (1 H, dd, / = 8.0, 0.8 Hz), 7.67 (1 H, brs); 13CNMR ((CD3)2SO) delta 17.56, 113.59, 114.17, 122.99, 126.61, 132.67, 148.21, 171.73. |
| 87% |
With ammonium chloride; benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; N-ethyl-N,N-diisopropylamine; In N,N-dimethyl-formamide; at 20℃; for 60h;Inert atmosphere; |
Step A: To 2-amino-3-methylbenzoic acid (1.5 g, 10 mmol) in DMF (5 mL) at rt were added hydroxybenzatriazole (2.0 g, 13 mmol), 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide (2.3 g, 12 mmol), ammonium chloride (2.3 g, 42 mmol), and diisopropylethylamine (7.5 ml, 42 mmol). The mixture was purged with N2 and stirred for 60 h. The mixture was poured into water and extracte with EtOAc (50 mL×3), and the combined extracts were washed with brine (20 mL×2), dried over MgSO4, filtered, and concentrated under reduced pressure. The residue was dissolved in DCM and purified by silica gel chromatography eluting with 1:1 EtOAc/hexanes to afford 2-amino-3-methyl-benzamide as a white solid (1.3 g, 87%). |
| 64% |
|
1161) A 500 mL round bottom flask was charged with 2-amino-3-methylbenzoic acid (Combi-Blocks, 4.0 g, 26.5 mmol, 1 eq), NH4Cl (4.25 g, 79.4 mmol, 3 eq) and a stir bar. The flask was evacuated and back-filled with Ar (×3). Anhydrous DCM (100 mL) and anhydrous DMF (20 mL) were added. The resulting stirred mixture was cooled to 0 C. 1-hydroxybenzotriazole hydrate (3.93 g, 29.1 mmol, 1.1 eq) was added followed by 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (5.56 g, 29.1 mmol, 1.1 eq) 5 min later. After 1 h DIPEA (32 mL, 23.9 g, 185 mmol, 7 eq) was added dropwise. The reaction was stirred at 0 C. to room temperature overnight. The volatiles were removed via rotary evaporation. The residue was diluted with water and adjusted to pH?8-9 with conc. NH3(aq). The resulting mixture was extracted with EtOAc (×3). The combined organics were dried over Na2SO4. The solids were filtered off, and the volatiles were removed via rotary evaporation. The residue was diluted with DCM and the resulting mixture was diluted with hexanes. The solids were collected via vacuum filtration. The filter cake was triturated with 10% DCM in hexanes and dried. 2.56 g (17.0 mmol, 64% yield) of 135 was collected as an off-white solid. Mass spectrum (ESI+): m/z=151 [M+1]observed. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping