|
|
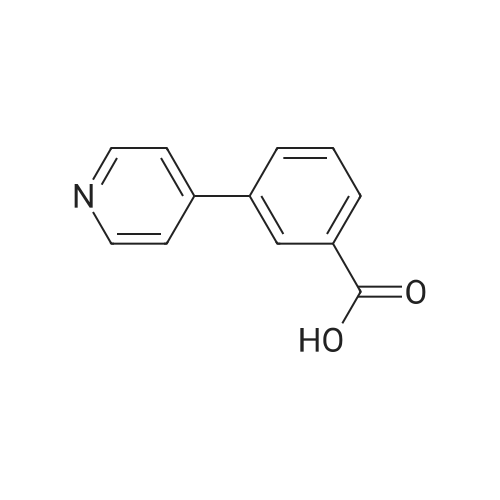
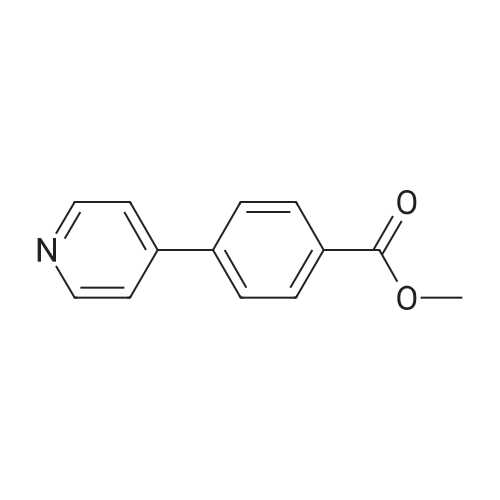
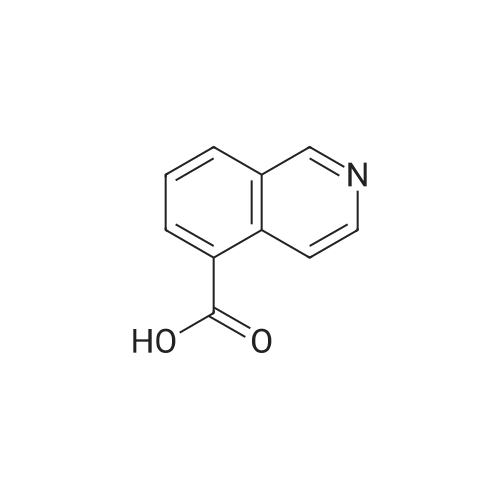
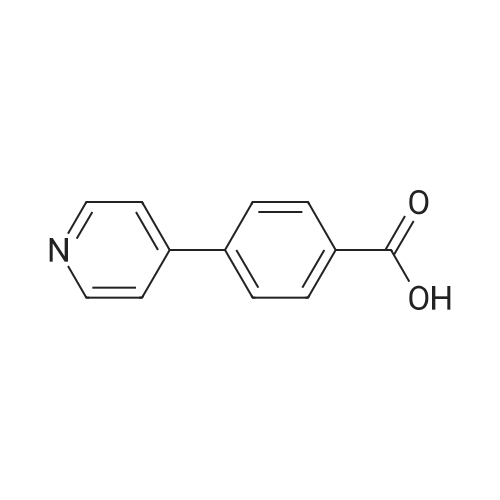
A solution of 1-(5-chlorobenzimidazol-2-ylsulphonyl)-4-(t-butyloxycarbonyl)piperazine (860 mg, 2.15 mmol) in dichloromethane/methanol (15 ml of 1:1) was treated with an excess of hydrogen chloride gas as a saturated solution in ethyl acetate. After stirring for 4 hrs. the solvent was removed in vacuo and the residue dried under high vacuum. This was then suspended in DMF and treated sequentially with 4-(4-pyridyl)benzoic acid (428 mg, 2.15 mmol), triethylamine (0.6 ml, 4.3 mmol) and 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (EDAC, 495 mg, 2.68 mmol). After stirring overnight the solvent was removed in vacuo and the residue taken up in dichloromethane (50 ml). This was washed sequentially with water, saturated sodium bicarbonate solution, water and brine. Evaporation of the solvent gave a residue which was purified by chromatography (MPLC on Merck Art 9385 silica, gradient eluting with ethyl acetate containing 0-8.0percent methanol) to give 1-(5-chlorobenzimidazol-2-ylsulphonyl)-4-[4-(4-pyridyl)benzoyl]piperazine as colourless crystals (370 mg) from ethanol, m.p. 242-244° C., 1H NMR (d6DMSO) 3.0-3.4 ppm (broad s, 4H), 3.4-3.8 ppm (broad s, 4H), 7.4 ppm (d, 1H), 7.5 ppm (d, 2H), 7.6-7.8 ppm (m, 4H), 7.85 ppm 2H), 8.6 ppm (d, 2H), 14.0 ppm (broad s, 1H); MS (M+H)+ 482/484. [00116] The requisite 1-(5-chlorobenzimidazol-2-ylsulphonyl)-4-(t-butyloxycarbonyl)piperazine starting material was prepared as follows. A suspension of 5-chloro-2-thiolbenzimidazole (500 mg, 2.71 mmol) in acetic acid (2.5 ml) and water (10 ml) was cooled to 5° C. and chlorine gas bubbled in slowly, keeping the temperature below 7° C. The flow of chlorine was maintained until no more was absorbed, and then for a further 15 mins., after which time the reaction was purged with argon. The suspension was filtered off, washed quickly with water and then added in small portions to a stirred, cooled (5° C.) solution of N-Boc piperazine (1.26 g, 6.78 mmol) in dichloromethane (20 ml). After stirring for 1 hr. At ambient temperature, the reaction mixture was diluted with more dichloromethane (30 ml) and washed sequentially with citric acid solution (30 ml, 1M), sat. brine (30 ml), water (2.x.30 ml) and sat. brine (30 ml). The solution was dried (Phase-Sep paper) and evaporated to give 1-(5-chlorobenzimidazol-2-ylsulphonyl)4-(t-butyloxycarbonyl)piperazine as a brown foam (880 mg, 81percent yield), which was used without further purification; 1H NMR (CDCl3) 1.4 ppm (s, 9H), 3.4 ppm (m, 4H), 3.6 ppm (m, 4H), 7.4 ppm (d, 1H), 7.4-7.6 ppm (broad s, 1H), 7.7-7.9 ppm (broad s, 1H); MS (M+H)+ 401/403 (w), (M+H-56)+ 345/347 (s). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping