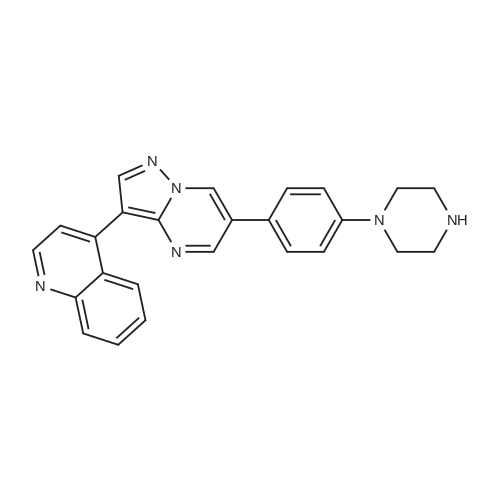
| 69% |
|
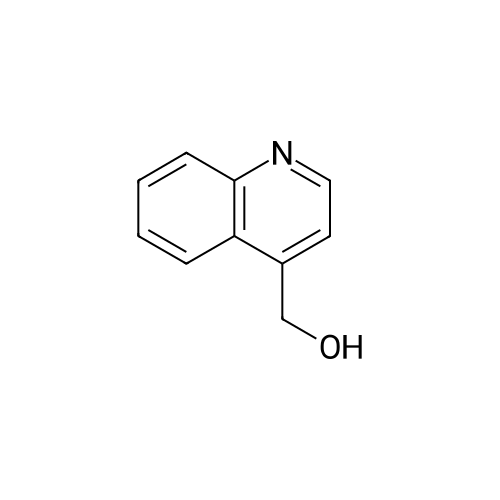
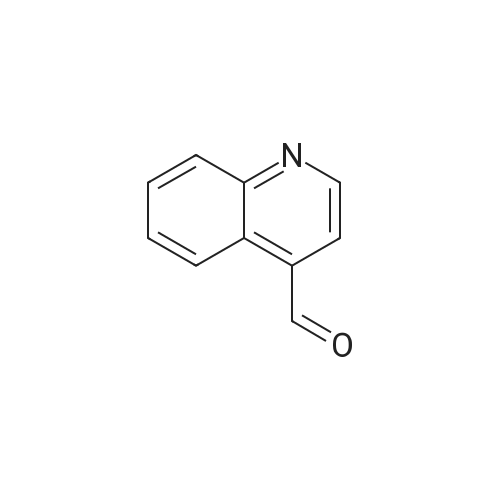
Reference Example 3 4-(Hydroxymethyl)quinoline (Reference Compound No.3-1); A solution of 4-quinolinecarboxylaldehyde (20g, 130mmol) in anhydrous tetrahydrofuran (200mL) was added dropwise for 30 minutes to a suspension of sodium borohydride (5.3g, 140 mmol) in anhydrous tetrahydrofuran (300mL) under ice-cooling, and the mixture was stirred for 1 hour at room temperature. Water (300mL) was added to the mixture, and the whole was then extracted with ethyl acetate (400mL x once, 100mL x three times). The organic layer was washed with brine (200mLx3 times) and dried over anhydrous magnesium sulfate. The solvent was evaporated under reduced pressure, and the resulting solid was filtered off with diethyl ether and washed to give 14g of the title Reference Compound as an orange-white solid (Yield: 69%) [Show Image] 1H-NMR (500MHz, DMSO-d6) delta 5.04 (dd, J = 5.5, 0.9 Hz, 2H), 5.57 (t, J=5.5 Hz, 1H), 7.57-7.63 (m, 2H), 7.76 (m, 1H), 8.02-8.06 (m, 2H), 8.70 (d, J = 4.3 Hz, 1H) |
| 65% |
With sodium tetrahydroborate; ethanol; at 0 - 20℃; |
To a solution of 4-quinolinecarboxaldehyde (0.47 g, 3.00 mmol) in a mixture of EtOH:THF=1:1 (40 mL) at 0 C. in a 100 mL round-bottomed flask equipped with a magnetic stirrer was added NaBH4 (0.11 g, 3.01 mmol) and the mixture was stirred overnight at RT. The solution was then cooled down to 0 C. before quenching a 6 N aq. HCl solution (2 mL). The reaction mixture was stirred at RT for 15 min then basified with a 2 N aq. NaOH solution (6 mL). EtOH was removed at 40 C. under vacuum and the residue was extracted with CH2Cl2 (2*50 mL). The organic phase was washed with brine (10 mL), dried over Na2SO4, filtered and concentrated at 40 C. under vacuum. Purification by column chromatography (SiO2, eluent cyclohexane:EtOAc=100:0 to 0:100) gave, after evaporation and drying, quinolin-4-ylmethanol MDE 32014 as an off-white solid (311 mg, 65% yield). MW: 159.19; Yield: 65%; Off-white solid; Mp ( C.): 109.3 Rf: 0.25 (EtOAc=100%). 1H-NMR (CDCl3, delta): 3.86 (broad s, 1H, OH), 5.23 (s, 2H, OCH2), 7.51-7.56 (m, 2H, 2*ArH), 7.69 (dd, 1H, J=7.6 Hz, ArH), 7.94 (d, 1H, J=8.4 Hz, ArH), 8.09 (d, 1H, J=8.4 Hz, ArH), 8.67-8.77 (m, 1H, ArH). 13C-NMR (CDCl3, delta): 61.3, 118.1, 122.9, 125.8, 126.7, 129.3, 129.7, 146.8, 147.6, 150.2. MS-ESI m/z (% rel. Int.): 160 ([MH]+, 100). |
| 64% |
With sodium tetrahydroborate; In water; |
Step a) Preparation of 4-Quinolinemethanol A solution of 4-quinolinecarboxaldehyde (55.0 g, 35 mmol) in ether (1 L) was added dropwise over 0.25 hours to a mechanically stirred biphasic solution of NaBH4 (13.24 g, 35 mmol), water (500 mL), and ether (100 mL) at 0 C. After 15 minutes, a second equivalent of NaBH4 (13.24 g, 35 mmol) dissolved in water was added, and the reaction allowed to warm to room temperature over 0.25 hour. The aqueous layer was separated, extracted with CH2 Cl2 (2*1 L), and the combined organic layer washed with water (500 mL), dried (MgSO4), filtered, and evaporated to a solid residue. Crystallization from methylene chloride-ether-hexane afforded 21.7 g (13.6 mmol, 39%) of a white solid. The filtrate was concentrated, and following HPLC separation, crystallization afforded 14.1 g (8.9 mmol, 25%) of a second crop, (35.8 g, 64% total yield), m.p. 95 -97 C. 1 H NMR (CDCl3, 400 MHz) delta: 8.79 (d, J=4.4 Hz, 1H), 8.10 (d, J=8.4 Hz, 1H), 7.94 (dd, J=8.4, 0.8 Hz, 1H), 7.70 (td, J=8.3, 1.4 Hz, 1H), 7.55 (td, J=8.5, 1.2 Hz, 1H), 7.54 (d, J=4.7 Hz, 1H), 5.23 (s, 2H), 3.80 (bs, 1H) MS (EI), m/z (rel. intensity)=159 (M+, 58), 130 (100) IR (KBr) v: 3280, 2830, 1585 cm-1 Anal. Calcd. for C10 H9 NO: C, 75.45; H, 5.70; N, 8.80. Found: C, 75.70; H, 5.65; N, 8.84. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping