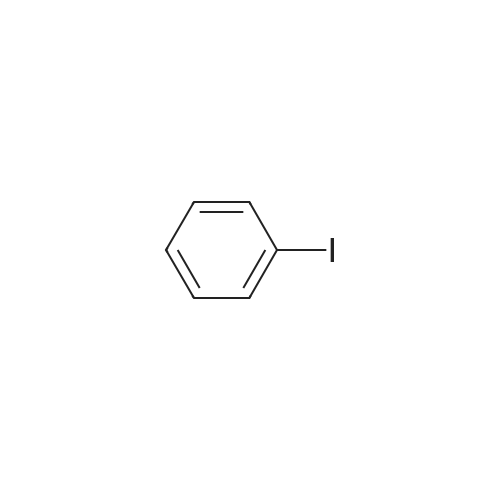
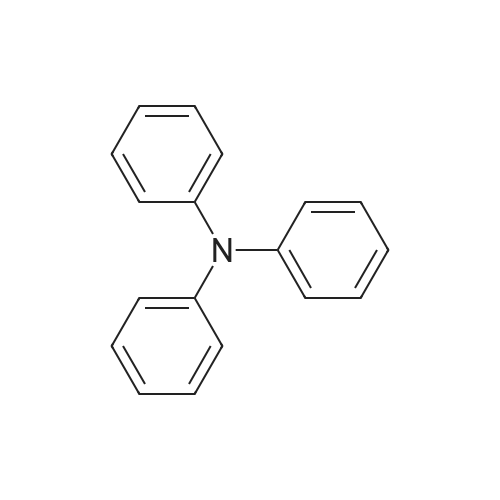
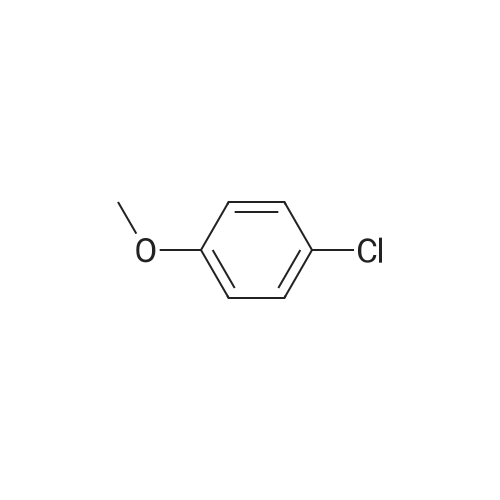
| 95% |
With sodium t-butanolate;palladium diacetate; P(i-BuNCH2)3CMe; In toluene; at 100℃; for 15 - 20h;Product distribution / selectivity; |
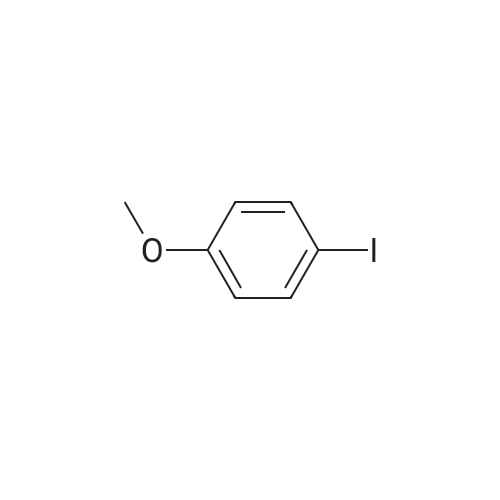
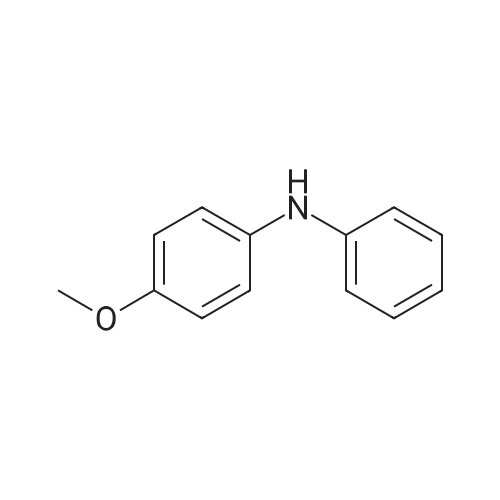
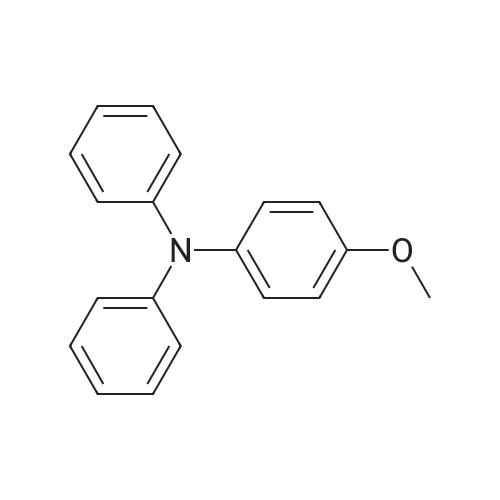
Pd(OAc)2/2-Catalyzed Amination of Aryl and Heteroaryl Bromides (Table 3).General Procedure: An oven-dried Schlenk flask equipped with a magnetic stirring bar was charged with Pd(OAc)2 (x mol %, see Table 3) and NaO-t-Bu (1.5 mmol). Amine (1.2 mmol) and aryl bromide (1.0 mmol) were also added at this time, if they were solids. The flask was capped with a rubber septum, evacuated and then flushed with argon. This cycle was repeated three times. Ligand 2 (2x mol %, see Table 3) was then added via syringe from a stock solution. Aryl bromide (if a liquid, 1.0 mmol), amine (if a liquid, 1.2 mmol) and toluene (3 mL) were then successively added by syringe. The reaction mixture was heated at the temperature indicated in Table 3 until the starting material had been completely consumed as judged by TLC (15-20 hours). The mixture was then cooled to room temperature, adsorbed onto silica gel and then purified by column chromatography (hexanes/ethyl acetate as eluent). |
| 95% |
With sodium t-butanolate;palladium diacetate; ?di-tert-butyl(2,2-diphenyl-1-methyl-1-cyclopropyl)phosphine; In toluene; at 100℃; for 3h;Product distribution / selectivity; |
Under a nitrogen atmosphere, diphenylamine (0.85g, 5.0mmol), and biphenyl as an internal standard were placed in a reaction flask, followed by addition of 10ml of toluene. To the mixture were added sodium tert-butoxide (0.58g, 6.0mmol), 4-bromoanisole (0.69ml, 5.5mmol), palladium acetate (2.8mg, 0.0125mmol) and 2,2-diphenyl-1-(di-tert-butylphosphino)-1-methylcyclopropane (8.8mg, 0.025mmol) obtained in Example 4 and the resulting mixture was stirred at 100C for 3 hours. The reaction mixture was cooled and analyzed gas chromatography to reveal the formation of the objective diphenyl (4-methoxyphenyl) amine in a yield of 95%. 1H-NMR (CDCl3) delta 3.80 (s, 3H), 6.79-7.28 (m, 14H) |
|
|
In a 100 ml flask purged with a nitrogen atmosphere,30 g of o-dichlorobenzene, 2.54 g (15 mmol) of diphenylamine,0.48 g (19 mmol) of sodium hydride,3.68 g (20 mmol) of magnesium bromide was charged,The reaction solution was heated to 135 C. while stirring.After aging for 2 hours at the same temperature,0.035 g (0.1 mmol) of iron (III) acetylacetonate,1.87 g (10 mmol) of bromoanisole was added,Further aging was carried out for 14 hours at the same temperature. After completion of the reaction,After cooling, water was added to dissolve the salt and liquid separation was carried out. After separating the organic layer,As a result of analysis by GC using the internal standard method,4- (N, N-diphenylamino) anisole as a target product was produced in a yield of 88%. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping