|
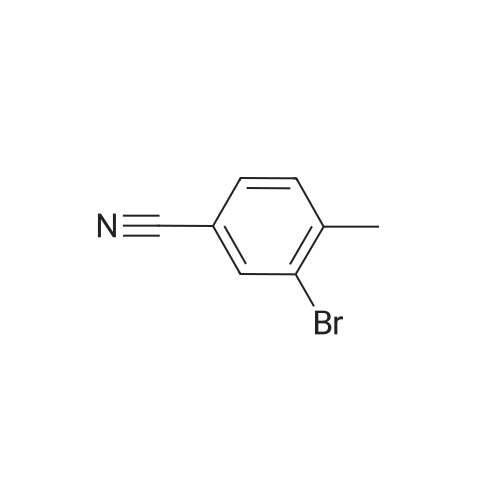
With N-Bromosuccinimide; dibenzoyl peroxide; In tetrachloromethane; for 16h;Reflux; Inert atmosphere; |
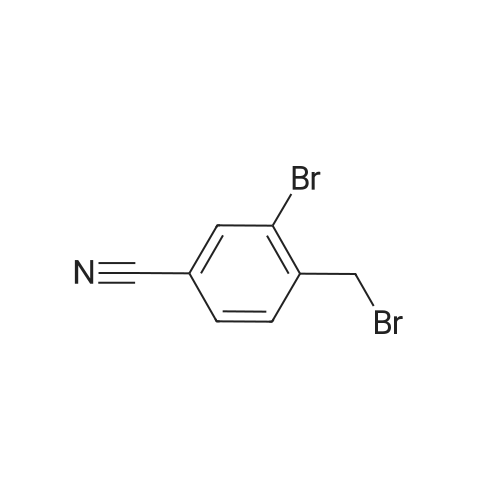
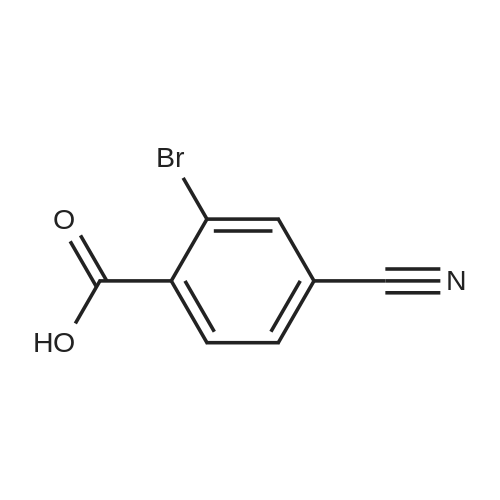
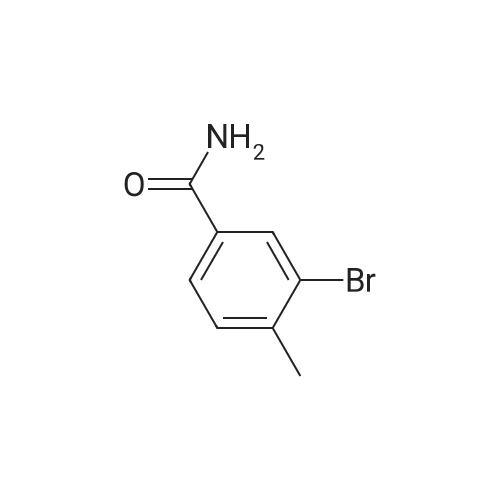
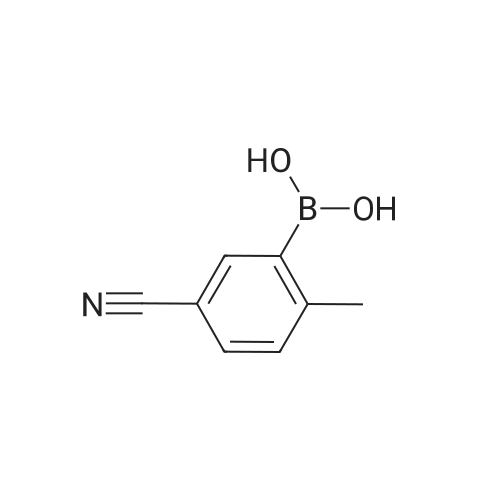
General procedure: To 2-bromo-3-methylbenzoic acid (21, 159 g, 739 mmol) in dichloromethane (1000 mL) was added triethylamine (TEA, 119.7 mL, 813 mmol, 1.1 equiv) followed by iso-butyl chloroformate (101.5 mL, 813 mmol, 1.1 equiv) in dichloromethane (DCM, 200 mL) at 0 C over 10 min. Concentrated ammonia water (323 mL) was then added at 0 C over 2 min. The reaction mixture was poured into water (200 mL), cooled to rt and filtered. The solid was washed with water (2 × 300 mL), 0.5 N HCl (2 × 150 mL) and dried to give the amide as a solid (120 g, yield 76%). To the solution of the amide obtained (50 g, 233.6 mmol) in DMF (300 mL) was added 2,4,6-trichloro-1,3,5-triazine (64.6 g, 350.4 mmol, 1.5 equiv) dropwise at 0 C and the reaction was stirred at rt overnight. To the reaction was added 600 mL of water and the reaction was stirred for 30 min. All insoluble was removed by filtration and the solid was triturated with ethyl acetate (EA, 3 × 100 mL) for 40 min and filtered. The filtrate was washed with saturated sodium carbonate (3 × 200 mL), saturated sodium chloride (200 mL) and dried over anhydrous sodium sulfate.The solvent was removed under reduced pressure to give 22 as a solid (42 g, yield 87.3%). To a solution of 22 (20 g, 102.2 mmol, 1 equiv) in CCl4 (200 mL) was added NBS (18.2 g, 102.0 mmol, 1.0 equiv), Bz2O2 (0.15 g, 0.6 mmol, 0.006 equiv). The reaction was refluxed overnight under N2, cooled and filtered. The filtrate was crystallized at 0 C to provide the brominated intermediate (15 g, yield 53.6%). To a solution of the brominated intermediate (90 g, 327.3 mmol) in DMF (760 ml) was added KOAc (38.6 g, 393.1 mmol, 1.2 equiv). The reaction mixture was stirred at 80 C for 1 h, cooled and water (1 L) was added. The mixture was extracted with EA (1 L). The organic layer was washed with 0.5 N HCl (3 ×200 mL), 2% NaHCO3 (200 mL) and dried over anhydrous sodium sulfate. The solvent was removed to give 23 as a yellow solid (77.3 g, yield 92.9%). 1H NMR of 23 (500 MHz, CDCl3): delta 2.16 (s, 3H), 5.21 (s, 2H), 7.43-7.46 (t, 1H,), 7.63 (m, 2H) ppm. To a solution of 23 (20 g, 78.8 mmol) in 1,4-dioxane (400 mL) was added bis(pinacolato)diboron (30 g, 118.1 mmol, 1.5 equiv) and KOAc (33.2 g, 338.1 mmol, 4.3 equiv). After being de-gassed and backfilled with nitrogen, Pd(dppf)Cl2 (3.2 g, 3.935 mmol, 0.05 equiv) was added. The reaction was refluxed overnight under nitrogen, cooled and filtered. The filtrate was concentrated and the residue was purified by silica gel column chromatography eluted with petroleum ether (PE)/EA = 5:1 to give 24 as red oil (29 g, crude yield 100% with 80% purity). 1H NMR of 24 (500 MHz, DMSO-d6): delta 1.42 (s, 12H), 2.20 (s, 3H), 5.25 (s, 2H), 7.44-7.49 (t, 1H), 7.57-7.64 (m, 2H) ppm. To a solution of 24 (29 g) in MeOH (100 mL) was added a solution of NaOH in MeOH (7.0 g/130 mL, 175.8 mmol, 2.3 equiv) and the reaction was stirred for 2 h at rt. The reaction mixture was concentrated under vacuum and the residue was dissolved in THF (150 mL) and 2 N HCl (138 mL, 69 mmol, 0.9 equiv). The reaction was stirred at rt for 50 min, concentrated and filtered. The solid was washed with water (3 × 20 mL) and petroleum ether (3 × 20 mL) to provide 25 (7.6 g, yield 62%). 1H NMR of 25 (500 MHz, DMSO-d6): delta 5.05 (s, 2H), 7.63-7.68 (t, 1H), 7.73-7.81 (m, 2H) ppm. To Raney Ni (0.849 g, 14.5 mmol, 2.3 equiv) in formic acid (10 mL) and water (2 mL) was added 25 (1 g, 6.29 mmol) at rt. The reaction was stirred at 100 C for 1 h, cooled and then filtered. The solvent was removed to give a solid that was purified by silica gel column chromatography eluted with CH2Cl2 to give 26 as a solid (0.714 g, yield 70%). 1H NMR of 26 (500 MHz, CDCl3): delta 10.03 (s, 1H), 8.08 (s, 1H), 7.86 (t, 1H), 7.63-7.71 (m, 2H), 5.20 (s, 2H) ppm. To a mixture of HCOOH (116.2 g, 10.0 equiv) and TEA (102.2 g, 4.0 equiv) were added 26 (40.9 g, 252.5 mmol) and 2,2-dimethyl-1,3-dioxane-4,6-dione (43.7 g, 1.2 equiv). The resulting mixture was refluxed for 15 h and cooled to rt. Hydrochloric acid (2 N, 320 mL) was added into the mixture that was then extracted with ethyl acetate twice (2 × 250 mL). The combined organic layer was washed with 2 N HCl (160 mL) and rotary evaporated to give the crude product that was recrystallized from DMF and 2 N HCl (34:204 mL) providing compound 1 as a white solid (15.6 g, yield 30%). An additional recrystallization from DMF and 2 N HCl (16:96 mL) was performed to give high purity product (12.9 g). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping