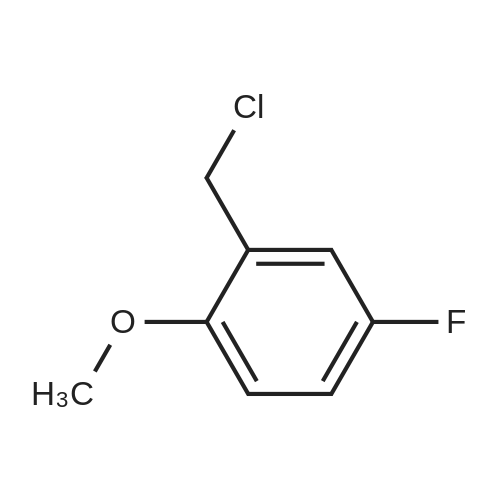
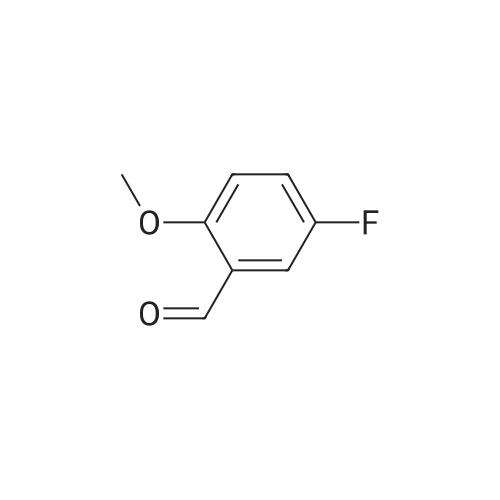
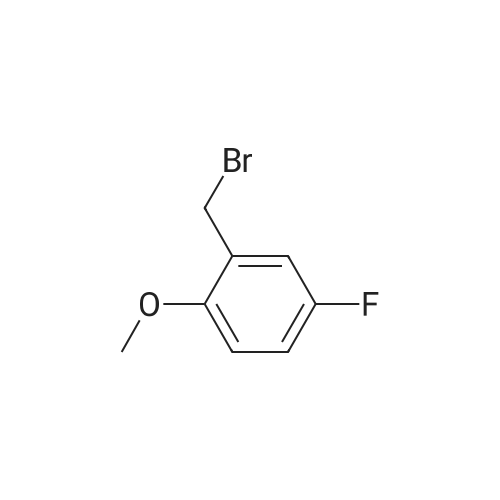
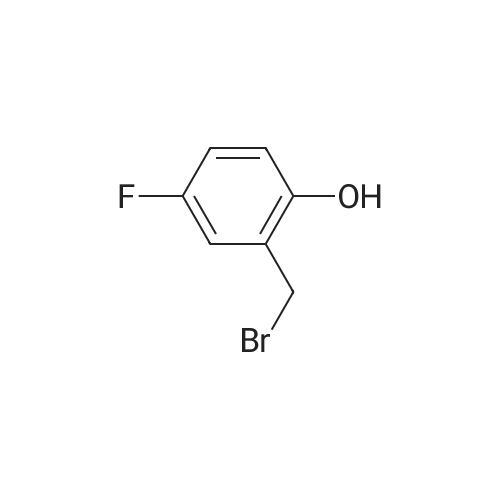
| 87% |
|
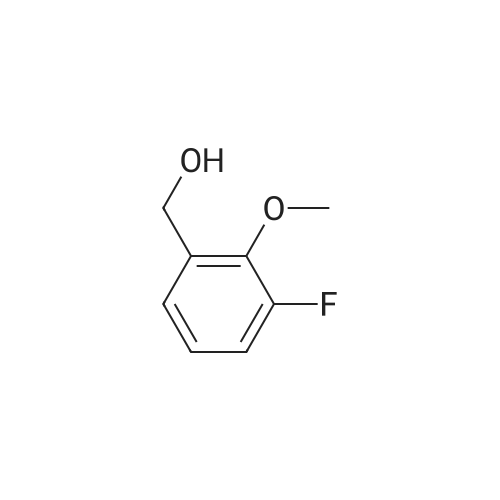
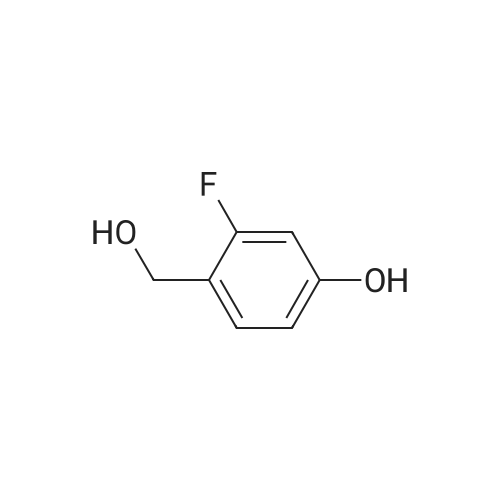
To a solution of 2-Methoxy-5-fluorobenzaldehyde (11. 093g, 1 equiv.-available from Aldrich Chemical Company) in methanol at-10 C under nitrogen atmosphere was added NaBH4 (7.515g, 2.7 equiv. ) portionwise. The solution was allowed to warm to room temperature and after 30 minutes the reaction solvent was removed under reduced pressure and replaced with dichloromethane. This solution was poured onto ice water and further extracted with dichloromethane. The organic fractions were collected and dried (MgSO4) and the solvent removed under reduced pressure to give the title compound as an oil (9.794g, 87%).'H NMR (300MHz, CDC13) : S 2.58 (m, 1H), 3.81 (s, 3H), 4.63 (d, 2H, J = 6.3 Hz), 6.78 (dd, 1H, J = 8.9 and 4.3 Hz), 6.94 (td, 1H, J = 8. 5 and 3. lHz), 7.04 (dd, 1H, J= 8. 7 and 3. 1Hz). |
| 52% |
With sodium tetrahydroborate; In methanol; at -10 - 20℃; for 0.5h; |
(5-Fluoro-2-methoxy-phenyl)-methanol To a solution of <strong>[19415-51-1]2-methoxy-5-fluorobenzaldehyde</strong> (11. 093g, 1 eq, available from Aldrich Chemical Company) in methanol at-10 C under nitrogen atmosphere is added NaBH4 (7. 515g, 2.7 equiv. ) portionwise. The solution is allowed to warm to room temperature and after 30 minutes the reaction solvent is removed under reduced pressure and replaced with dichloromethane. This solution is poured onto ice water and further extracted with dichloromethane. The organic fractions are collected and dried (MgS04) and the solvent removed under reduced pressure to give the title compound as an oil (9.794g, 87%). MW 156.16 ; CsH9F02 ;'H NMR (CDC13) : 2. 58 (m, 1H), 3.81 (s, 3H), 4.63 (d, 2H, 6.3 Hz), 6.78 (dd, 1H, 8. 9 Hz and 4.3 Hz), 6.94 (td, 1H, 8.5 Hz and 3.1 Hz), 7.04 (dd, 1H, 8.7 Hz and 3.1 Hz). |
|
With sodium tetrahydroborate; In ethanol; at 20℃; for 1h; |
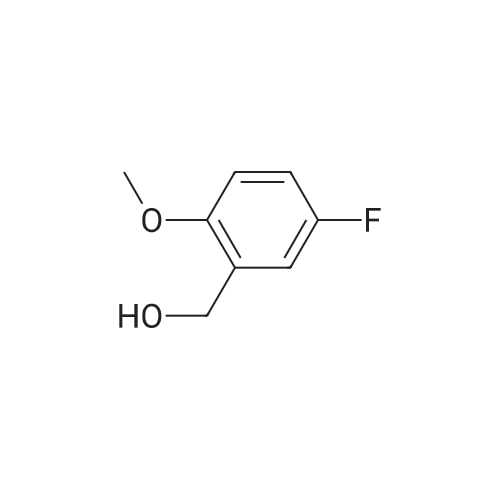
This intermediate is synthesised as follows: Add Sodium borohydride (540 mg, 13.95 mmol) in portions to a solution of 5-Fluoro-2- methoxy-benzaldehyde (2.15g, 13.94 mmol) in absolute EtOH (20 ml) and stir at room temperature. After lh, evaporate the solvent, dilute the residue in CH2Cl2 and treat with aqueous 3M HCI. Separate the phases, wash the organic one twice with H2O, dry over Na2S04 and concentrate at vacuum to obtain pure (5-Fluoro-2-methoxy-phenyl)-methanol as white solid. Add aqueous concentrated HBr (15 ml) to a solution of (5-Fluoro-2- methoxy-phenyl)-methanol (1.9g, 12.17 mmol) in CHC13 (10 ml) and stir at room temperature. After 1h, separate the phases, wash the aqueous one with CH2C12, combine organic phases, wash with H2O, dry over Na2SO4 and concentrate at vacuum to obtain a residue. Purify the residue by column chromatography on silica gel eluting with hexane to afford 2-Bromomethyl-4-fluoro-1-methoxy-benzene as white solid.'H-NMR (CDCl3, 200 MHz) : 8 7.06 (dd, J=3.0 and 8.6 Hz, 1H), 6.98 (m, 1H), 6.81 (dd, J= 4.4 and 9.0 Hz, 1H), 4.50 (s, 2H), 3.87 (s, 3H). |
|
With lithium aluminium tetrahydride; In tetrahydrofuran; at 20 - 60℃; |
To a suspension of LiAlH4 (1.2 g, 32 mmol) in dry THF (5 mL) was added the aldehyde (10 mmol) and the mixtures were stirred at room temperature for two hours. Mixtures with aldehydes as starting materials were put aside and the acids were heated at 60 C. overnight. To each mixture was added in consecutive order water (1.2 mL), 2 M aqueous NaOH (1.2 mL), and water (3.6 mL). The precipitate was filtered off and the solvent was removed under reduced pressure to yield the target products as oils. The title compound was prepared starting from <strong>[19415-51-1]5-fluoro-2-methoxybenzaldehyde</strong> and was obtained as a light red oil (94% yield). Fragmenting MS analysis supports the stated structure. Purity 97% (GC). 1H NMR (CDCl3) ?3.28 (s, 3 H), 4.64 (s, 2 H), 6.78 (m, 1 H), 6.93 (m, 1 H), 7.02 (m, 1 H). 13C NMR (CDCl3) ?55.73, 61.34, 110.83 (d, J=8.5 Hz), 114.22 (d, J=22.6Hz), 115.26 (d, J=23.3 Hz), 138.68 (d, J=6.4 Hz), 153.18 (d, J=2.1 Hz), 156.95 (d, J=238.8 Hz). |
|
With sodium tetrahydroborate; In ethanol; at 0℃; for 1h; |
A 100ml four-necked flask was charged with 5.0 g (32.4 mmol) of <strong>[19415-51-1]5-fluoro-2-methoxybenzaldehyde</strong> and 100ml of ethanol, and 1.23 g (32.4 mmol) of sodium borohydride was added thereto with stirring under ice-cooling.. Then, the mixture was stirred for 1 hour and allowed to stand at room temperature overnight.. After concentrating under reduced pressure, 50 ml of ethyl acetate and 50 ml of water were added to the residue, and the mixture was shaken and separated into layers.. The aqueous layer was extracted once again with 50 ml of ethyl acetate.. The ethyl acetate layers were combined, and washed with 50 ml of water followed by 50 ml of saturated brine.. After drying over anhydrous sodium sulfate, the mixture was concentrated under reduced pressure to obtain 5-fluoro-2-methoxybenzyl alcohol.1H-NMR (CDCl3) delta: 3.83 (s, 3H), 4.66 (d, 2H, J= 6.4Hz), 6.77-7.07 (m, 3H). |
|
With methanol; sodium tetrahydroborate; at 20℃; for 18h; |
Step 1: To 42 (1 eq, 17.1 mmol, 2.6 g) in MeOH (68 ml_) at room temperature, added NaBH4 {1.2 eq, 20.5 mmol, 775 mg) and the reaction mixture stirred 18h, then concentrated in vacuo. The crude residue was diluted with ethyl acetate, washed with 1 N aqueous HCI then brine, dried over sodium sulfate, and concentrated in vacuo to give 43 (2.8 g) as a pale yellow oil |
|
|
a) Preparation of (5-fluoro-2-methoxy-phenyl)-methanolSodium borohydride (61 mg, 1.62 mmol) is added to a solution of 5-fluoro-2- methoxybenzaldehyde (1.0 g, 6.49 mmol) in MeOH (10 mL). After 1 hour, the reaction is poured in to 1 M aqueous HCI and extracted with DCM. The combined organic phases are dried (Na2SO4) , evaporated and purified by flash chromatography (0-100% EtOAc- isohexanes gradient elution) to afford (5-fluoro-2-methoxy-phenyl)-methanol. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping