| 69% |
|
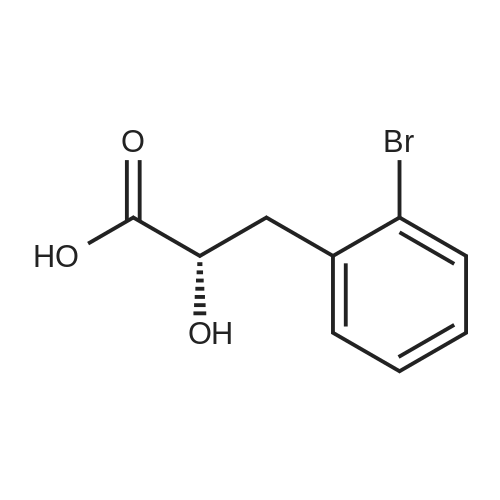
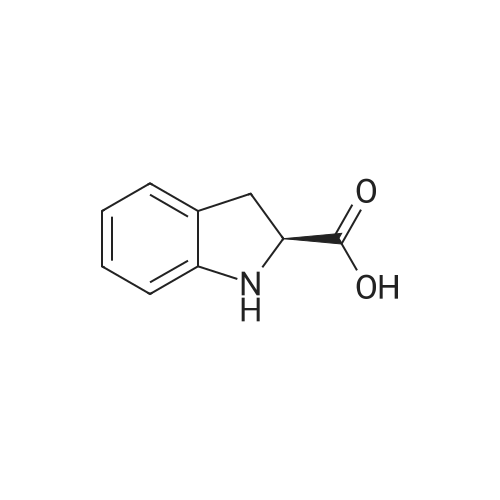
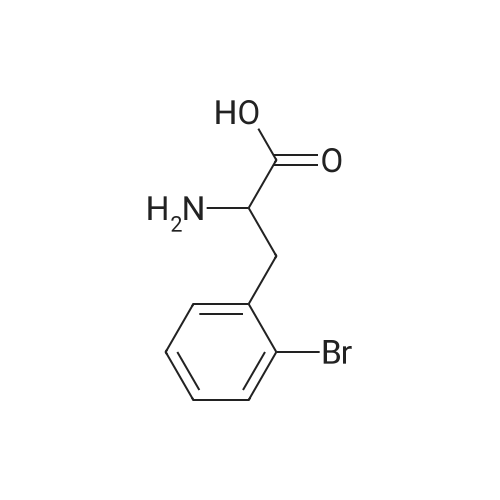
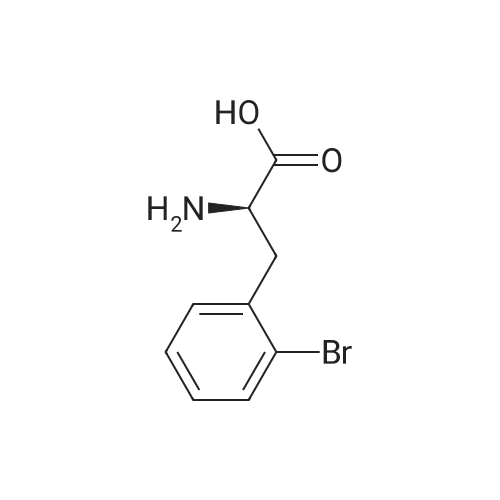
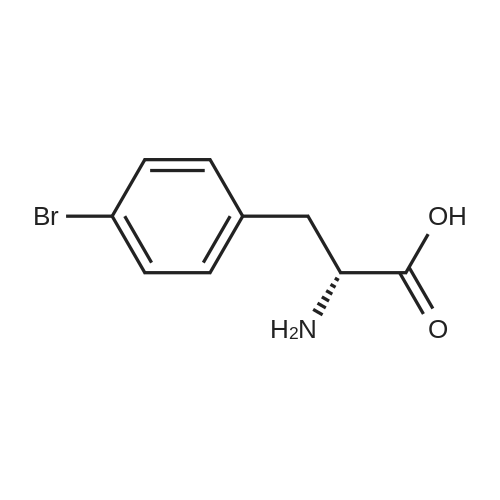
Example 4; (S)-2,3-dihydro-1 H-indole-2-carboxylic acid: Conversion of (S)-2- bromophenylalanine with 0.01 molpercent CuCI in water at 95°C EPO <DP n="30"/>; A flask was charged successively with 4.89 g (20.0 mmol) (S)-2- bromophenylalanine, 2.93 g (21.2 mmol) K2CO3, 0.2 mg CuCI (2.9 mmol) and 39.7 g H2O. The reactor was flushed with argon and then kept under a slow stream of argon. The reaction mixture was stirred and heated until 95°C and kept at this temperature. Samples were taken regularly and analyzed by HPLC. After 4h hour full conversion of <strong>[42538-40-9](S)-2-bromophenylalanine</strong> was found. The reaction mixture was cooled to 25°C. Then the reaction mixture was acidified with 4.47 g 5M aqueous HCI until pH = 4.4. The precipitated (S)-2,3-dihydro-1H-indole-2-carboxylic acid was isolated by filtration and washed with 2 x 10 ml_ H2O. Found after drying 2.24 g (13.7 mmol) (S)-2,3-dihydro-1H- indole-2-carboxylic acid. Yield 69percent, ee >99percent. |
| 49.5% |
|
Example 2; (S)-2,3-dihydro-1H-indole-2-carboxylic acid: Conversion of (S)-2- bromophenylalanine with 1 molpercent CuCI in NMP at 800C; A flask was charged successively with 9.76 g (40.0 mmol) (S)-2- bromophenylalanine, 5.80 g (42.0 mmol) K2CO3, 40 mg (0.4 mmol) CuCI and 40 g NMP. The reactor was flushed with argon and then kept under a slow stream of argon. The reaction mixture was stirred and heated until 800C and kept at this temperature. Samples were taken regularly and analyzed by HPLC. After 3.5 h full conversion of (S)- 2-bromophenylalanine was found. The reaction mixture was cooled to 25°C and then 40 ml_ H2O and 50 mL aqueous EtOAc were added. The pH of this mixture was adjusted to 3.3 with 3.5 g 37percent aqueous HCI. The phases were separated. The H2O phase was extracted with 2 x 50 mL aqueous EtOAc. The combined organic layers were washed with 25 mL sat aqueous NaCI. Then the organic phase was concentrated. The residu was dissolved in 16 mL 5N HCI, followed by pH adjustment to 2.1 with 9.4 g 32percent aqueous NaOH. The precipitated (S)-2,3-dihydro-1H-indole-2- carboxylic acid was isolated by filtration and washed with 2 x 10 mL H2O. 3.24 g (19.8 mmol) (S)-2,3-dihydro-1 H-indole-2-carboxylic acid was found after drying. Yield 49.5percent, ee >99percent. |
| 95.9%Chromat. |
With potassium carbonate; copper(l) chloride; In 1-methyl-pyrrolidin-2-one; at 100℃; for 4h;Product distribution / selectivity; |
Examplei; (S)-2,3-dihydro-1H-indole-2-carboxylic acid: Conversion of <strong>[42538-40-9](S)-2-bromophenylalanine</strong> with 2 molpercent CuCI in NMP at 1000C; A flask was charged successively with 366 mg (1.5 mmol) (S)-2- bromophenylalanine, 217 mg (1.6 mmol) K2CO3, 3.2 mg (0.03 mmol) CuCI and 3.2 g NMP. The reactor was flushed with argon and then kept under a slow stream of argon.The reaction mixture was stirred and heated until 1000C and kept at this temperature.Samples were taken regularly and analyzed by HPLC. After 4 hours, full conversion of<strong>[42538-40-9](S)-2-bromophenylalanine</strong> was found. The yield (measured in solution) of (S)-2,3- dihydro-1 H-indole-2-carboxylic acid was 95.9 percent, ee > 98.6percent. |
| 81.1%Chromat. |
With potassium carbonate; copper(l) chloride; In water; at 95℃; for 2h;Product distribution / selectivity; |
Example 3; (S)-2,3-dihydro-1 H-indole-2-carboxylic acid: Conversion of (S)-2- bromophenylalanine with 2 molpercent CuCI in water at 95°C; A flask was charged successively with 366 mg (1.5 mmol) (S)-2- bromophenylalanine, 217 mg (1.6 mmol) K2CO3, 3 mg (0.03 mmol) CuCI and 3.4 g H2O. The reactor was flushed with argon and then kept under a slow stream of argon. The reaction mixture was stirred and heated until 95°C and kept at this temperature. Samples were taken regularly and analyzed by HPLC. After 2 hour full conversion of <strong>[42538-40-9](S)-2-bromophenylalanine</strong> was found. The reaction mixture was cooled to 25°C and analyzed by HPLC. The yield in solution of (S)-2,3-dihydro-1H-indole-2-carboxylic acid was 81.1 percent, ee > 99percent. |
| 95.6%Chromat. |
With potassium carbonate; In water; at 95℃; for 22h;Product distribution / selectivity; |
Example 5; (S)-2,3-dihydro-1H-indole-2-carboxylic acid: Conversion of (S)-2- bromophenylalanine without CuCI in water at 95°C; A flask was charged successively with 366 mg (1.5 mmol) (S)-2- bromophenylalanine, 217 mg (1.6 mmmol) K2CO3 and 3 g H2O. The reactor was flushed with argon and then kept under a slow stream of argon. The reaction mixture was stirred and heated until 95°C and kept at this temperature. Samples were taken regularly and analyzed by HPLC. After 5h hour the conversion was approximately 37percent. After 22h full conversion of <strong>[42538-40-9](S)-2-bromophenylalanine</strong> was found. The reaction mixture was cooled to 25°C and analyzed by HPLC. The yield in solution of (S)-2,3-dihydro-1 H- indole-2-carboxylic acid was 95.6 percent, ee > 99percent. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping