|
In dichloromethane; |
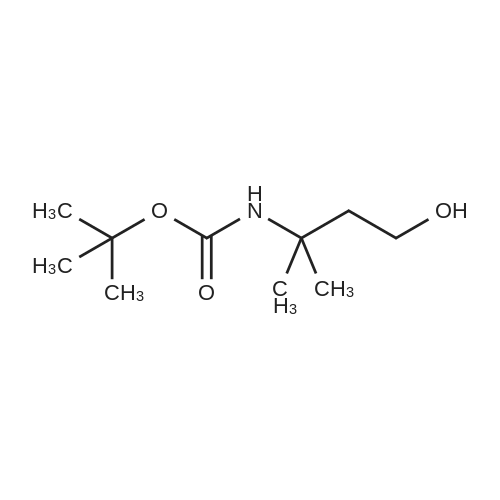
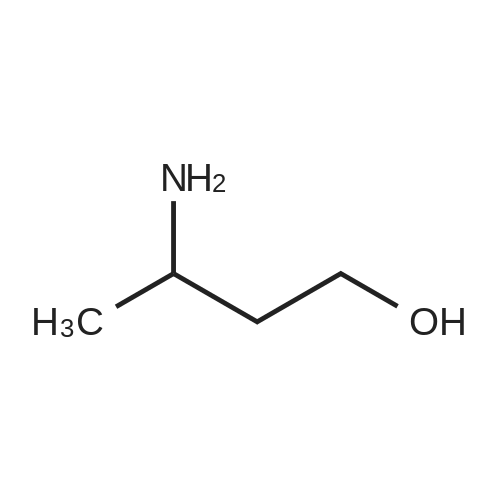
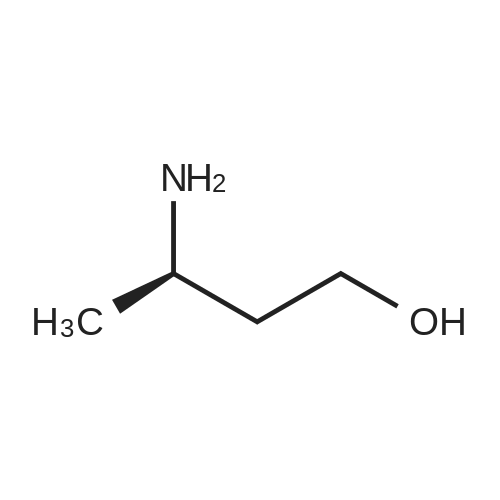
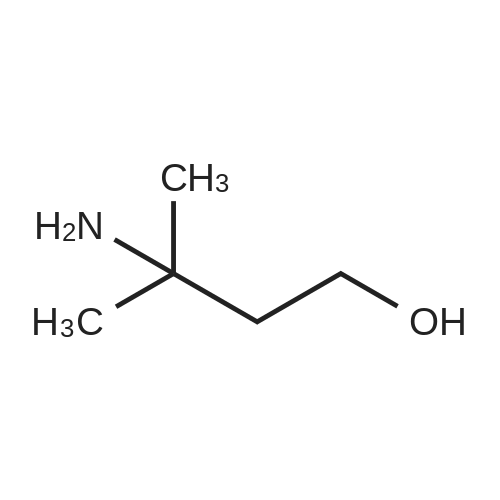
b 3-(N-BOC-amino)-3-methyl-butan-1-ol A solution of 24.88 g (0.114 mol) of di-tert-butyl dicarbonate in 70 ml of methylene chloride is added dropwise to a solution of 11.2 g (0.1086 mol) of 3-amino-3-methyl-butan-1-ol [Z. Naturforsch., Teil B, 38, 1146 (1983)] in 70 ml of methylene chloride and the mixture is stirred at room temperature for 90 hours. After evaporation of the reaction mixture in vacuo, the oily title compound is obtained as a crude product, Rf value=0.52 (silica gel/methylene chloride:methanol (9:1)). |
|
In dichloromethane; |
C (3-Hydroxy-1,1-dimethyl-propyl)-carbamic Acid Tert-Butyl Ester 3-Amino-3-methyl-butan-1-ol (5.0 g, 0.04 mol) was dissolved in 150 mL CH2Cl2 and treated with di-tert-butyl dicarbonate (11.12 g, 0.05 mol). The resulting mixture was stirred for 18 h at 25 C. The mixture was concentrated to the desired material as an amber oil (9.8 g, 100%): 1H NMR (DMSO-d6) δ 3.55 (s, 1H), 4.43 (t, 1H, J=5 Hz), 3.46 (m, 2H), 1.71 (t, 2H, J=7 Hz), 1.37 (s, 9H), 1.12 (s, 6H). |
|
In dichloromethane; |
Step 4: (3-Hydroxy-1,1-dimethyl-propyl)-carbamic acid tert-butyl ester. 3-Amino-3-methyl-butan-1-ol(5.0 g, 0.04 mol) was dissolved in 150 mL CH2Cl2 and treated with di-tert-butyl dicarbonate(11.12 g, 0.05 mol). The resulting mixture was stirred for 18 h at 25 C. The mixture was concentrated to the desired material as an amber oil(9.8 g, 100%); 1H NMR (DMSO-d6) δ 3.55 (s,1H), δ 4.43 (t, J=5 Hz, 1H), δ 3.46 (m, 2H), δ 1.71 (t, J=7 Hz, 2H), δ 1.37(s, 9H), δ 1.12(s, 6H). |
|
In ethyl acetate; for 1.5h; |
a. Tert-butyl (3-hvdroxy-1 ,1-dimethylpropyl)-carbamidate200 g (1.94 mol) 3-amino-3-methylbutan-1 -ol in 0.75 I ethyl acetate are combined with a solution of 435.0 g (1.99 mol) di-tert-butyl-dicarbonate in 0.75 I ethyl acetate within one hour. The reaction mixture is stirred for 30 min and the solvent is eliminated in vacuo. The residue obtained is used in the next step without further purification. Yield: 412.5 g1H-NMR (DMSO, 400 MHz): 1.19 (s, 9H); 1.36 (s, 6H); 1.68-1.74 (m, 2H); 3.42-3.50 (m, 2H); 4.39 (t, J = 4.8, 1 H); 6.36 (br s, 1 H).Alternatively, tert-butyl (3-hydroxy-1 ,1-dimethylpropyl)-carbamidate may also be prepared by the methods described for example in J. of Labell. Compounds & Radioph. 2001 , 44(4), 265-275 or WO 03/037327, p. 82/83. |
|
In ethyl acetate; for 1.5h; |
Component XI [preparation of the compound of formula (IIIb)]N-tert-butoxycarbonyl-4,4-dimethyl-[1,2,3]oxathiazinane-2,2-dioxide a. tert-butyl (3-hydroxy-1,1-dimethylpropyl)-carbamidate 3-amino-3-methylbutan-1-ol (200.0 g, 1.94 mol) is dissolved in ethyl acetate (0.75 l) and within one hour combined with a solution of di-tert-butyl-dicarbonate (435.0 g, 1.99 mol) in ethyl acetate (0.75 l). After the addition has ended the reaction mixture is stirred for another 30 min. After elimination of the solvent the title compound is obtained, which is used in the next step without further purification. Yield: 412.5 g 1H-NMR (DMSO, 400 MHz): 1.19 (s, 9H); 1.36 (s, 6H); 1.68-1.74 (m, 2H); 3.42-3.50 (m, 2H); 4.39 (t, J=4.8, 1H); 6.36 (br s, 1H). Alternatively tert-butyl (3-hydroxy-1,1-dimethylpropyl)-carbamidate may also be prepared using the methods described for example in J. of Labell. Compounds & Radioph. 2001, 44(4), 265-275 or der WO 03/037327, p. 82/83. |
|
In ethyl acetate; for 16h; |
Intermediate X: 3,3-dimethyl-1-oxo-2,3,4,5-tetrahydro-1H-[1,4]diazepino[1,2- a]indole-8-carboxylic acid Step 1: Synthesis of tert-butyl (4-hydroxy-2-methylbutan-2-yl)carbamate To a solution of 3-amino-3-methylbutan-1-ol (1.0 g, 9.7 mmol) in EtOAc (5 mL) is added di-tert-butyl dicarbonate (2.1 g, 9.7 mmol). The mixture is stirred for 16 h and the solvent is evaporated to afford the crude title compound which is used in the next step without purification |
|
In dichloromethane; at 20℃; for 18h; |
To a solution of 3-amino-3-methyl-butan-1-ol (5.0 g, 48.54 mmol) in CH2C12 (150 mL) was added di-tert-butyl dicarbonate (13.22 g, 60.27 mmol) at RT and resulting mixture was stirred for 18 h. Progress of reaction was monitored by TLC. After completion, reaction mixture was concentrated under vacuum to afford tert-butyl (4-hydroxy-2-methylbutan-2-yl)carbamate asan amber oil (9.8 g) which was used in the next step without purification. |
|
|
Synthesis of tert-butyl (4-hydroxy-2-methylbutan-2-yl)carbamate Into a 200-mL round-bottom flask under a nitrogen atmosphere, was placed a solution of 3-amino-3- methylbutan-1 -ol (2.5 g, 24.2 mmol, 1 equiv) and TEA (4.9 g, 48.5 mmol, 2 equiv) in anhydrous DCM (50 ml_). The mixture was stirred for 10 minutes at 25C. Then (Boc)20 (5.3 g, 24.2 mmol,1 equiv) was added in one portion. The resulting solution was stirred for 6 hours at 25 C. The reaction was then quenched by the addition of water (20 ml_). The resulting solution was extracted with dichloromethane (3x30 ml.) and the organic layers combined was washed with brine, dried over anhydrous sodium sulfate and concentrated in vacuum. This resulted in tert- butyl N-(4-hydroxy-2-methylbutan-2-yl)carbamate which was used in the next step directly. |
| 9.8 g |
In dichloromethane; at 20℃; for 18h; |
To a solution of 3-amino-3-methyl-butan-1-ol (5.0 g, 48.54 mmol) in CH2Cl2 (150 mL) was added di-tert-butyl dicarbonate (13.22 g, 60.27 mmol) at RT and resulting mixture was stirred for 18 h. Progress of reaction was monitored by TLC. After completion, reaction mixture was concentrated under vacuum to afford tert-butyl (4-hydroxy-2-methylbutan-2-yl)carbamate as an amber oil (9.8 g) which was used in the next step without purification. |
|
In dichloromethane; at 25℃; for 12h; |
tert-Butoxycarbonyl tert-butyl carbonate (1.3 g, 5.8 mmol, 1.3 mL, 1.2 eq) and 3-amino-3-methyl-butan-1-ol (0.5 g, 4.8 mmol, 1 eq in DCM (15 mL) was stirred at 25C for 12 hr. The reaction mixture was concentrated under reduce pressure to give tert-butyl (4- hydroxy-2-methylbutan-2-yl)carbamate |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping