| 97% |
With potassium carbonate; In ethanol; water; for 24h;Reflux; |
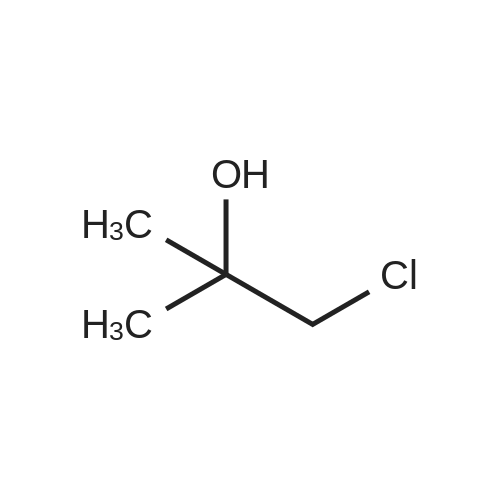
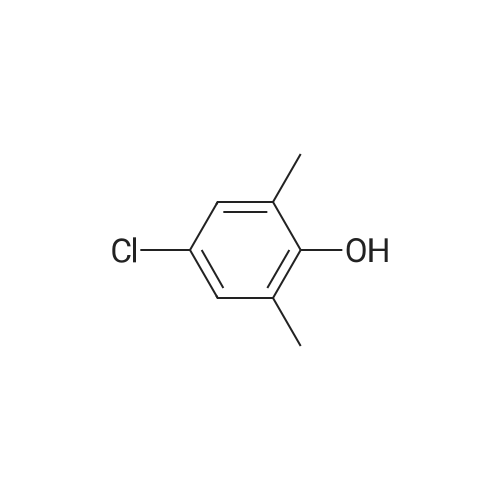
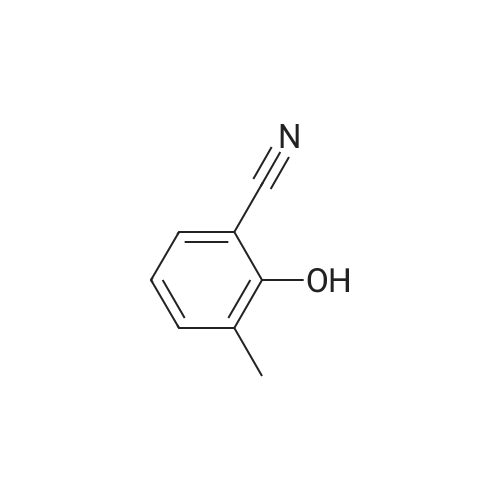
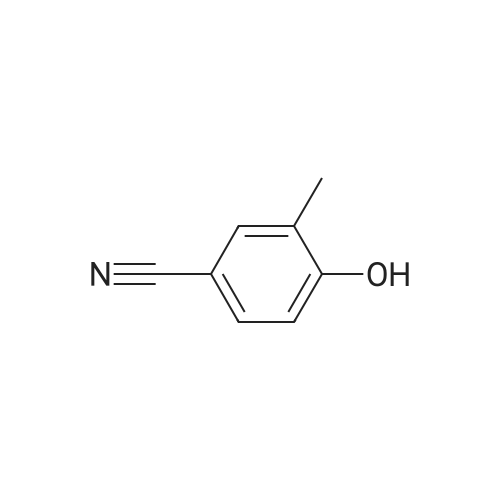
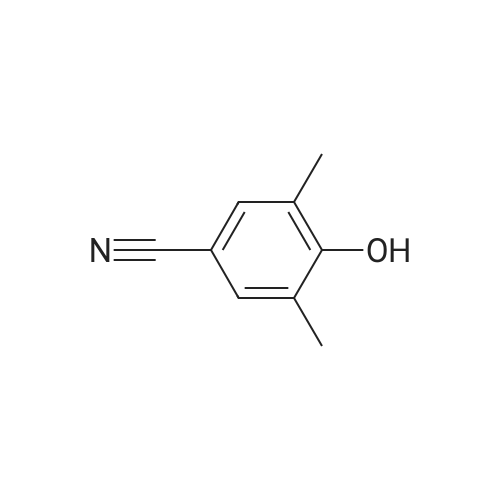
4-hydroxy-3,5-dimethyl-benzonitrile (2.00g, 13.5mmol) and 1-chloro-2-methyl-2-ol (8.85g, 81.5mmol) in ethanol(50 mL) of was added potassium carbonate (7.5g,54mmol) and water (5mL). The reaction mixture was stirred at reflux for 24 hours,cooled to room temperature. The precipitated solid was filtered off, washedwith water. The solid was dissolved in ethyl acetate (100 mL) in water (50 mL),brine (50 mL), dried over anhydrous Na 2SO 4dry. The solvent was removed togive 4- (2-hydroxy-propoxy) -3,5-dimethyl-benzonitrile(2.9g, 97percent) as a white solid. ? 4-(2-hydroxy-2-methyl-propoxy) -3,5-dimethyl-benzonitrile (2.90g, 13.2mmol) wasadded imidazole (2.7g in dry DMF (20mL) in a solution, 40mmol) andtert-butyldimethylsilyl chloride (2.19g, 14.6mmol). At room temperature, thereaction mixture was stirred under nitrogen for 3 days. Was added water(200mL), and the mixture was extracted with ethyl acetate (200mL). The organiclayer was washed with water (2 × 100mL) and brine (100 mL), dried over anhydrousNa 2SO 4dry. The solvent was removed under reduced pressure and the crudecompound was purified by column chromatography to give 4- [2-(tert-butyldimethylsilyloxy) -2-methyl-propoxy] -3,5 methyl-benzonitrile(2.24g, 54percent). At -10 deg.] C, over 10 minutes, under nitrogen n-butyllithium(6.2mL, 6.6mmol, 1.6M solution in hexane) was added dropwise to 2,4-dimethoxy-6-N- dimethyl-benzamide (0.9g, 4.3mmol) in anhydrous THF (10mL) in a solution.Stirring was continued at 0 1 hour. Thereaction mixture was cooledto -50 . Rapidly added 4- [2-(tert-butyldimethylsilyloxy) -2-methyl-propoxy] -3,5-dimethyl-benzonitrile(1.58g, 4.73mmol) in dry THF ( 5mL) was added. The cooling bath was removed andthe reaction mixture was allowed to warm to room temperature. Stirring was continued at room temperaturefor 1 hour. Was added aqueous ammonium chloride solution (10mL), then ethylacetate (100mL). The organic layer was separated, which was washed with water(10 mL), dried (Na 2SO 4). The solvent was removed under reduced pressure, andthe crude compound by column chromatography (silica gel 230-400 mesh; used inCH 2Cl 20-5percent methanol as eluant) to afford a white solid 3- {4- [2-(tert-butyldimethylsilyloxy) -2-methyl propoxy] -3 ,5-dimethyl-6,8-dimethoxy-phenyl} -2H- isoquinolin-1-one (0.82g, 37percent). ? The abovecompound (0.42g, 0.82mmol) was dissolved in anhydrous THF (20mL) in. At 0 was added tetrabutylammonium fluoride (4.1mL,1.0M solution in THF). At 0 The reactionmixture was stirred for 10 minutes, then stirred at room temperature for 2 hours, then stirred at 70 24 hours. The mixture was cooled to roomtemperature. Saturated ammonium chloride solution (30mL). The organic layer wasseparated, which was washed with water, brine, dried over anhydrous Na 2SO 4dry. The solvent was removed underreduced pressure. The crude product was purified by column chromatography(silica gel 230-400 mesh; used in CH 2Cl 20-4percent methanol as eluant) to afford awhite solid of 3- (4- (2-hydroxy-propoxy) -3,5-dimethylphenyl) -6 , 8--dimethoxy isoquinoline -1 (2H) - one (0.15-g, 46percent). Selected data: MS (ES) m /z: 397.98; MP252-254 ,decomposition. |
| 97% |
With potassium carbonate; In ethanol; water; for 24h;Heating / reflux; |
To a solution of 4-hydroxy-3,5-dimethylbenzonitrile (2.00 g, 13.5 mmol) and 1-chloro-2-methyl propan-2-ol (8.85 g, 81.5 mmol) in ethanol (50 mL) was added potassium carbonate (7.5 g, 54 mmol) and water (5 ml_). The reaction mixture was stirred at reflux for 24 h and cooled to RT. The precipitated solid was filtered off and washed with water. The solid was dissolved in ethyl acetate (100 mL), washed with water (50 mL), brine (50 mL), and dried over anhydrous Na2SCU. Removal of solvent gave 4-(2-hydroxy-2-methylpropoxy)-3,5- dimethyl benzonitrile (2,9 g, 97percent) as a white solid.[0113] To a solution of 4-(2-hydroxy-2-methylpropoxy)-3,5-dimethyl benzonitrile (2.90 g, 13.2 mmol) in anhydrous DMF (20 mL) was added imidazole (2.7 g, 40 mmol) and terf-butyldimethylsilylchloride (2.19 g, 14.6 mmol). The reaction mixture was stirred at RT under nitrogen for 3 d. Water (200 mL) was <n="60"/>added and the mixture was extracted with ethyl acetate (200 mL). The organic layer was washed with water (2x100 mL) and brine (100 mL), and dried over anhydrous Na2SO4. The solvent was removed under reduced pressure and the crude compound was purified by column chromatography to give 4-[2-(tert- butyldimethylsilanyloxy)-2-methylpropoxy]-3,5-dimethylbenzonitrile (2.24 g, 54percent). n-Butyl lithium (6.2 mL, 6.6 mmol, 1.6 M solution in hexanes) was added to a solution of 2,4-dimethoxy-6-/v"-dimethylbenzarnide (0,9 g, 4.3 mmol) in anhydrous THF (10 mL) drop-wise at -10°C over a period of 10 rnin under nitrogen. The stirring was continued at 0"C for 1 h. The reaction mixture was cooled to -50°C. A solution of 4-[2-(tert-butyidimethylsilanyloxy)-2-methylpropoxy]-3,5- dimethylbenzonitrile (1.58 g, 4,73 mmol) in anhydrous THF (5 mL) was quickly added. The cooiing bath was removed and the reaction mixture was allowed to warm to RT. The stirring was continued at RT for 1 h. An aqueous ammonium chloride solution (10 mL) was added followed by ethyl acetate (100 mL). The organic layer was separated, washed with water (10 mL) and dried (Na2SO4). The solvent was removed under reduced pressure and the crude compound was purified by column chromatography (silica gel 230-400 mesh; 0-5percent methanol in CH2CI2 as eluent) to give 3-{4-[2-(tert- butyldimethylsilanyloxy)-2-methylpropoxy]- 3,5-dimethylphenyl}-6,8-dimethoxy-2H-isoquinolin-1-one (0.82 g, 37percent), as a white solid.[0114] The above compound (0.42 g, 0.82 mmol) was dissolved in anhydrous THF (20 mL). Tetrabutylammonium fluoride (4.1 mL, 1.0 M solution in THF) was added at 0°C. The reaction mixture was stirred at 0°C for 10 min, then at RT for 2 h and then stirred at 70°C for 24 h. The mixture was cooled to RT.Saturated aqueous ammonium chloride (30 mL) was added. The organic layer <n="61"/>was separated, washed with water, brine, and dried over anhydrous Na2SO4. The solvent was removed under reduced pressure. The crude product was purified by column chromatography (silica gel 230-400 mesh; 0-4percent methanol in CH2Cl2 as eluent) to give 3-(4-(2-hydroxy-2-methylpropoxy)-3,5-dimethylphenyl)- 6,8-dimethoxyisoquinolin-1(2H)-one (0.15-g, 46percent), as a white solid. Selected data: MS (ES) m/z: 397.98; MP 252-254 °C at decomposition. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping