|
With polyphosphoric acid; In 1,4-dioxane; at 130℃; for 5h;Inert atmosphere; |
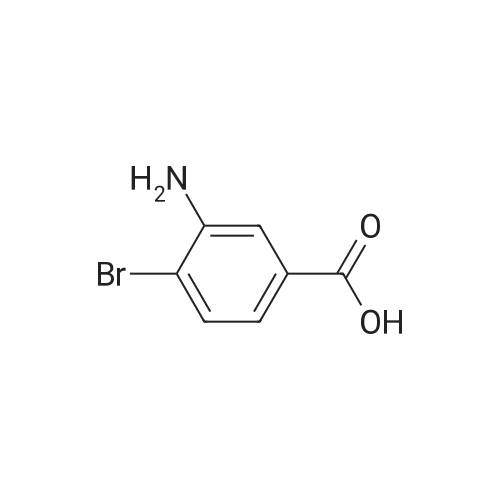
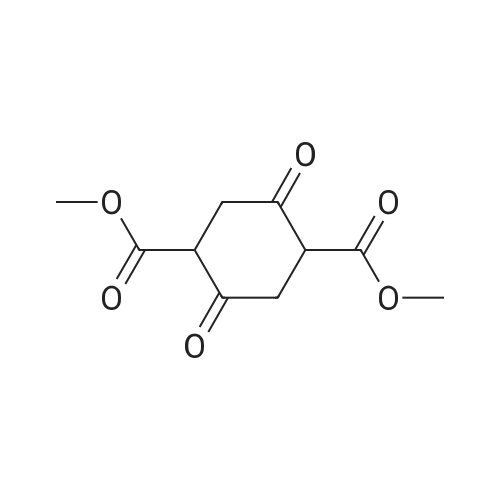
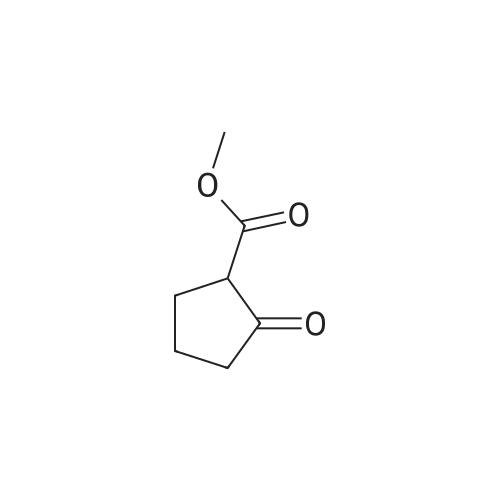
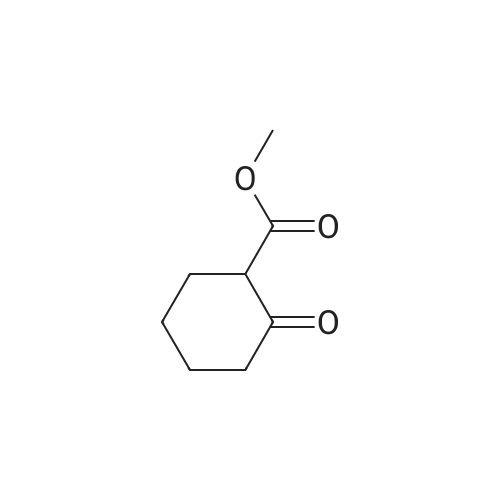
A mixture of methyl 2-oxocyclohexanecarboxylate (1.70 g, 10 mmol), <strong>[2840-29-1]3-amino-4-bromobenzoic acid</strong> (2.16 g, 10 mmol), polyphosphoric acid (15 g) and dioxane (12 mL) was heated at 130 C for 5 hours. After cooling to room temperature, NaOAc-3H2O (27 g) was added and the pH~3. Then the mixture was diluted with water and the resulting precipitate was collected by filtration and dried. The solid was suspended in methanol (80 mL) and SOCl2 (16 mL) was added at 0-15 C, the mixture was stirred at reflux for 5 hours. After cooling to room temperature, the mixture was concentrated and treated with water (100 mL), extracted with ethyl acetate (3x100 mL), the organic layer was separated, and concentrated to afford the crude product. Then the mixture was purified by chromatography column on silica gel (eluted withCH2Cl2 /MeOH) to afford methyl 4-bromo-9-oxo-5,6,7,8,9,10-hexahydroacridine-1-carboxylatet (2.20 g). MS (ESI) m/e [M+1]+336. A mixture of methyl 4-bromo-9-oxo-5,6,7,8,9,10-hexahydroacridine-1-carboxylate (0.19 g, 0.56 mmol), MeOH (20 mL), and Pd/C (5% Pd on carbon, 50% water, 0.05 g) were stirred at room temperature under an atmosphere of hydrogen for 6 hours. The mixture was filtered through celite and the filtrate was concentrated to give crude methyl 9-oxo-5,6,7,8,9,10- hexahydroacridine-1-carboxylate (0.22 g), which was used to next step without further purification. To the solution of the crude methyl 9-oxo-5,6,7,8,9,10-hexahydroacridine-1-carboxylate in DMA (4 mL) was added hydrazine hydrate (4 mL) at room temperature, and the mixture was heated at 130 C for4.0 h. The reaction mixture was cooled to room temperature and stirred for another 12 hours. Then the mixture was filtered and recrystallized from MeOH (twice) to give the product (20 m g, 15%) as a yellow solid. |
|
With polyphosphoric acid; In 1,4-dioxane; at 130℃; for 5h; |
A mixture of methyl 2-oxocyclohexanecarboxylate (1.70 g, 10 mmol), <strong>[2840-29-1]3-amino-4-bromobenzoic acid</strong> (2.16 g, 10 mmol), polyphosphoric acid (15 g) and dioxane (12 mL) was heated at 130 C. for 5 hours. After cooling to room temperature, NaOAc.3H2O (27 g) was added and the pH?3. Then the mixture was diluted with water and the resulting precipitate was collected by filtration and dried. The solid was suspended in methanol (80 mL) and SOCl2 (16 mL) was added at 0-15 C., the mixture was stirred at reflux for 5 hours. After cooling to room temperature, the mixture was concentrated and treated with water (100 mL), extracted with ethyl acetate (3*100 mL), the organic layer was separated, and concentrated to afford the crude product. Then the mixture was purified by chromatography column on silica gel (eluted with CH2 Cl2/MeOH) to afford methyl 4-bromo-9-oxo-5,6,7,8,9,10-hexahydroacridine-1-carboxylatet (2.20 g). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping