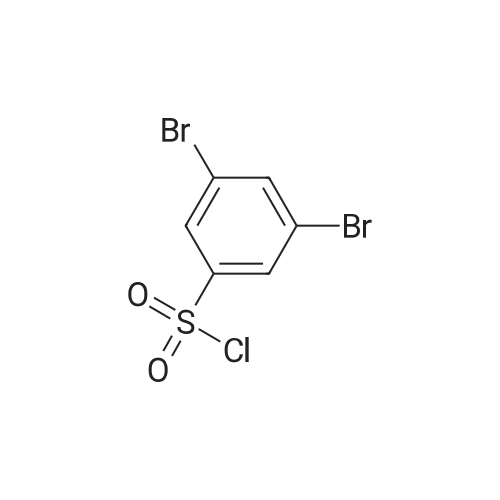
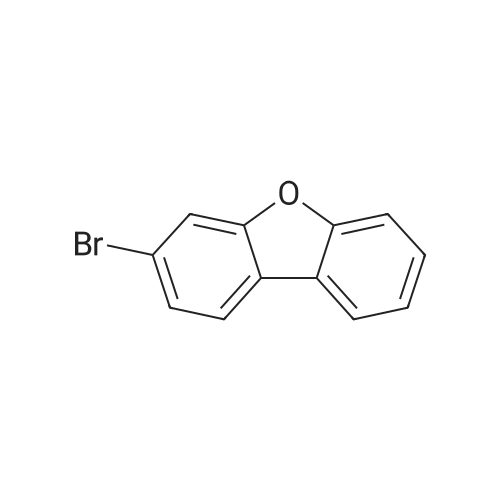
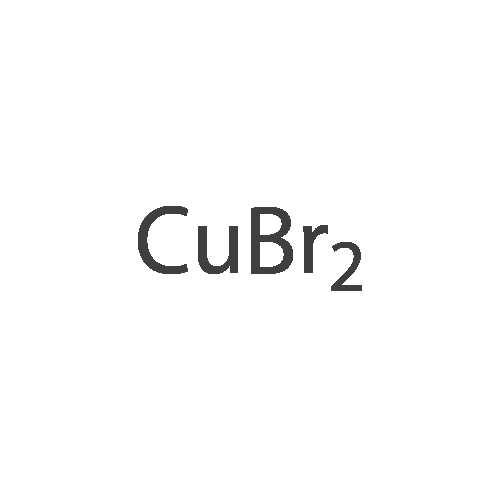| 68% |
|
To a 500 mL four-necked round bottom flask equipped with a stirrer, a Liebig condenser (not required), a thermometer, and a 200 mL dropping funnel, 19.0 g (102.6 mmol) of the amino compound obtained in the above ,190 mL of water and 57 mL of 48% hydrobromic acid were added and stirred overnight. Subsequently, the solution was cooled to -10 C. in an ice water bath, and a solution of 8.1 g (117.4 mmol) of sodium nitrite dissolved in 150 mL of water was added dropwise under conditions such that the temperature did not rise by 5 C. or more, and the diazotized The reaction solution was stirred at 5 C. or less for 1 hour to obtain a diazonium solution.Next, in a 1 L four-necked round bottom flask equipped with a stirrer, an Erlin condenser, a thermometer, and a 500 mL dropping funnel, 16.2 g (116.9 mmol) of cuprous bromide, 48% hydrobromic acid 38 mL and water 90 mL were added, and the mixture was cooled to 0 C. and stirred. Subsequently, the diazonium solution was added dropwise at a temperature not exceeding 5 C. and stirred at the same temperature for 30 minutes, then the temperature was raised to 40 C., and the mixture was stirred at the same temperature for 18 hours.200 mL of water was added to the obtained reaction solution, cooled to room temperature, and extracted twice with 200 mL of DCM. Then, the organic layer was washed with 100 mL of 5% sodium sulfite aqueous solution, then washed with 100 mL of saturated brine, dried with magnesium sulfate, magnesium sulfate was removed by suction filtration, and the solvent was distilled off under reduced pressure. Subsequently, the resulting crude product was purified by silica gel column chromatography using n-heptane as a developing solution to obtain 17.2 g (yield: 68.0%) of the desired bromide. |
| 67% |
|
Sodium nitrite (4.4 g, 65 mmol) was dissolved in 40 ml of concentrated sulfuric acid at 0 C, and compound M-2 (10.6 g, 58 mmol) was dissolved in a small amount of glacial acetic acid and slowly dropped into the reaction solution to maintain the temperature. Below 5 C, after the completion of the dropwise addition, the temperature was kept at 0 C and stirring was continued for 2 hours. Diethyl ether (200 ml) was added to the reaction mixture for stirring, and a diazonium salt was precipitated and filtered to obtain a brown solid; another reaction flask was added with CuBr (12.5g, 87mmol), 48% HBr (300ml), Finally, the obtained brown diazonium salt is added, warmed to 66 C for 2 hours, cooled to room temperature, filtered, filter cake washed twice with water, the obtained solid Petroleum ether: Eluent of dichloromethane = 10:1 was passed through the column to afford intermediate (9.6 g, 67%). |
|
|
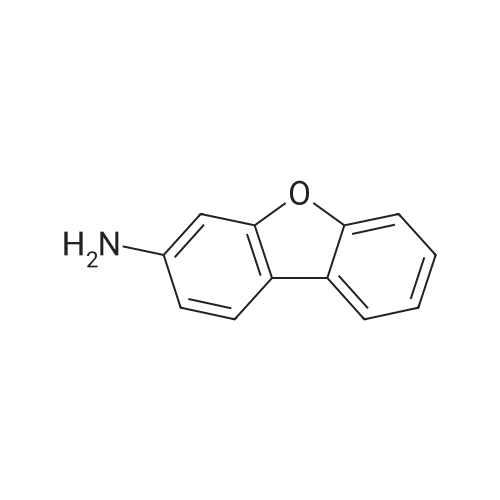
Example 11 3-Bromodibenzofuran Combine N-dibenzofuran-3-ylamine (2.0 g, 10.8 mmol), water (20 ml), and conc. hydrobromic acid (6 ml). Cool to 0 C. Add a solution of sodium nitrite (0.7 g, 10.8 mmol) in water (16 ml). After 15 minutes add the mixture above to a mixture of copper bromide (1.7 g, 12.3 mmol) in water (9.2 ml) and hydrobromic acid (4 ml). Warm to ambient temperature. After 18 hours, add water and extract with dichloromethane, Combine the organic layers and wash sequentially with distilled water and brine and then dry (Na2SO4), filter, and concentrate to give a residue. Chromatograph the residue eluting with 8:2 hexane:EtOAc to give the title compound. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping