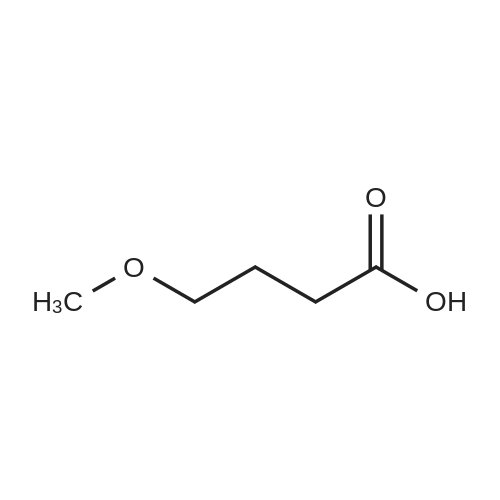
| 43% |
With polyphosphoric acid; at 120℃; for 1.5h; |
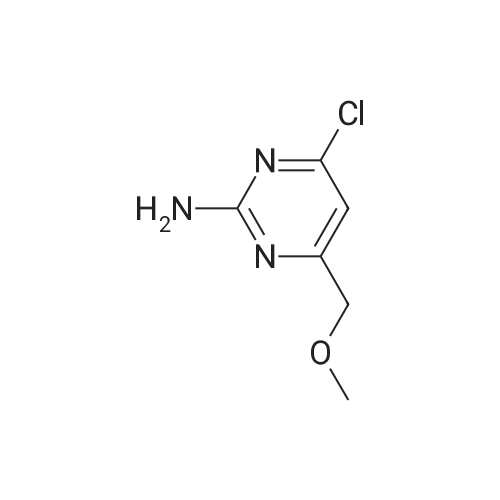
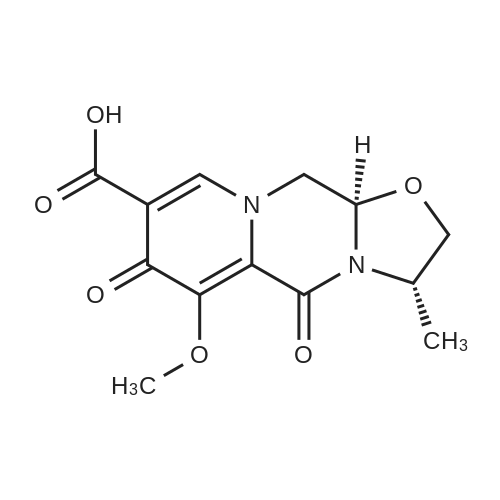
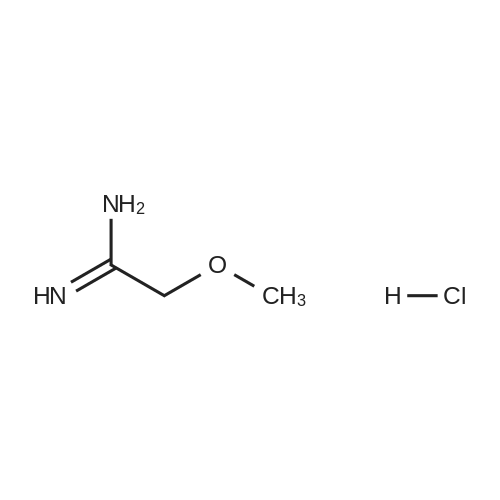
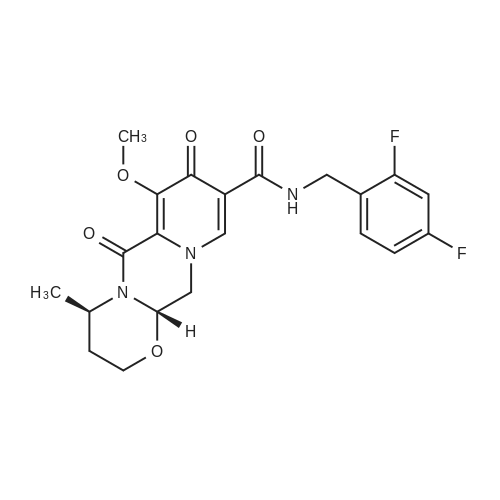
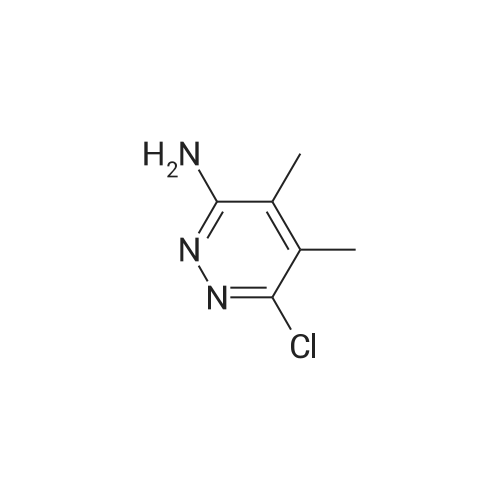
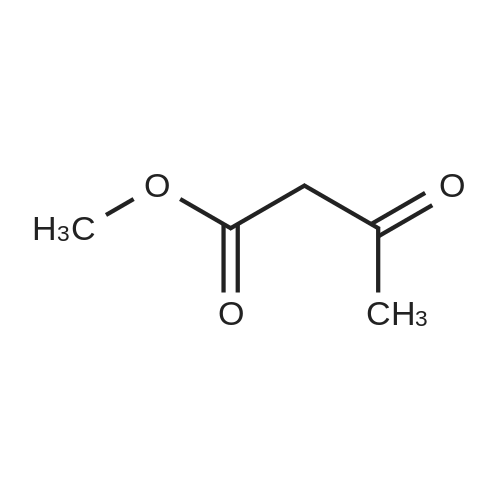
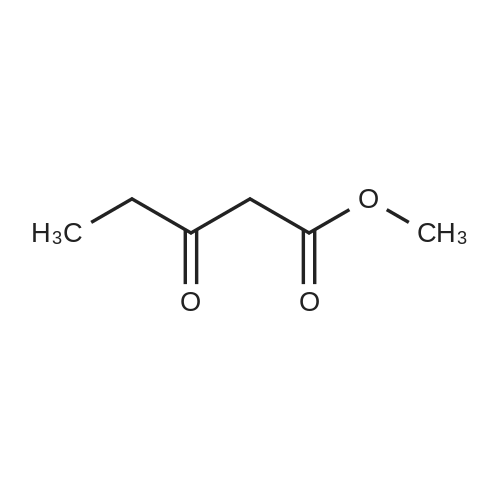
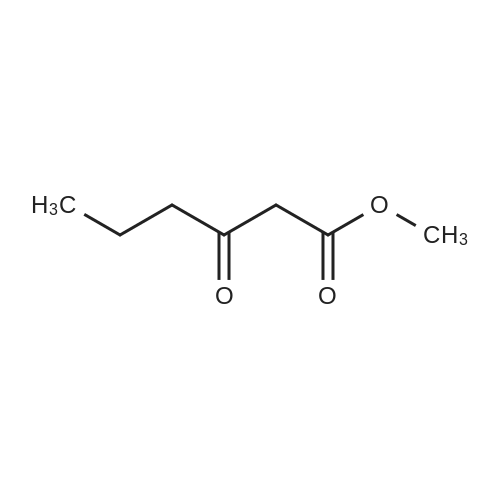
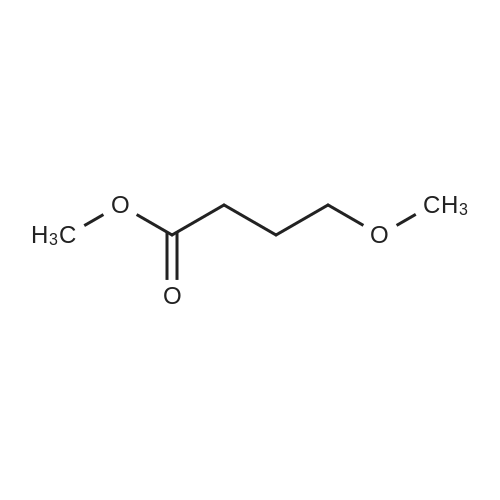
To a solution of 6-choro-3-amino-4,5-dimethylpyridazine (1 g) in polyphosphoric acid (9 mL) was added methyl 4-methoxy-3-oxo-butanoate (1.23 mL). The mixture was heated to 85 C for 1 hour, then 120 C for 30 minutes. The mixture was then quenched by slow addition to a solution of saturated sodium bicarbonate (~150 L) and 4:1 chloroform/' PA (~50 L). The organic layer was isolated and the aqueous layer further extracted (x3). The organic layers were pooled, dried over magnesium sulfate, filtered, and concentrated. The crude product was dissolved in DMSO (9 mL) and solid was filtered off. The filtrate was purified using the Gilson (Basic, 50 x 250 mm column, 5 - 55% ACM/ 0.05% aqueous NH4OH, 16 min run). Fractions containing product were concentrated to give the title compound (219 g) as a solid. The solid was washed with hexanes then dissolved in DCM and purified using a Teledyne ISCG Combi-Flash system (liquid loading with DCM, 80G column, 0-2% MeQH/DCM/NhUOH, 10 min run). Fractions containing product were concentrated to give the title compound (682 mg; 43% yield) as a solid. *H NMR (400 MHz, CDCI3) 6 6.77 (t, J = 1.0 Hz, 1H), 4.45 (d, J = 1.0 Hz, 2H), 3.51 (s, 3H), 2.59 (d, J = 0 9 Hz, 3H), 2.49 (d, J = 1.0 Hz, 3H). ES-MS [M+l]+: 254. |
| 43% |
With polyphosphoric acid; at 120℃; for 1.5h; |
To a solution of 6-choro-3-amino-4,5-dimethylpyridazine (1 g) in polyphosphoric acid (9 mL) was added methyl 4-methoxy-3-oxo-butanoate (1.23 mL). The mixture was heated to 85 C for 1 hour, then 120 C for 30 minutes. The mixture was then quenched by slow addition to a solution of saturated sodium bicarbonate (~150 L) and 4:1 chloroform/' PA (~50 L). The organic layer was isolated and the aqueous layer further extracted (x3). The organic layers were pooled, dried over magnesium sulfate, filtered, and concentrated. The crude product was dissolved in DMSO (9 mL) and solid was filtered off. The filtrate was purified using the Gilson (Basic, 50 x 250 mm column, 5 - 55% ACM/ 0.05% aqueous NH4OH, 16 min run). Fractions containing product were concentrated to give the title compound (219 g) as a solid. The solid was washed with hexanes then dissolved in DCM and purified using a Teledyne ISCG Combi-Flash system (liquid loading with DCM, 80G column, 0-2% MeQH/DCM/NhUOH, 10 min run). Fractions containing product were concentrated to give the title compound (682 mg; 43% yield) as a solid. *H NMR (400 MHz, CDCI3) 6 6.77 (t, J = 1.0 Hz, 1H), 4.45 (d, J = 1.0 Hz, 2H), 3.51 (s, 3H), 2.59 (d, J = 0 9 Hz, 3H), 2.49 (d, J = 1.0 Hz, 3H). ES-MS [M+l]+: 254. |
| 43% |
With polyphosphoric acid; at 120℃; for 1.5h; |
To a solution of 6-choro-3-amino-4,5-dimethylpyridazine (1 g) in polyphosphoric acid (9 mL) was added methyl 4-methoxy-3-oxo-butanoate (1.23 mL). The mixture was heated to 85 C for 1 hour, then 120 C for 30 minutes. The mixture was then quenched by slow addition to a solution of saturated sodium bicarbonate (~150 L) and 4:1 chloroform/' PA (~50 L). The organic layer was isolated and the aqueous layer further extracted (x3). The organic layers were pooled, dried over magnesium sulfate, filtered, and concentrated. The crude product was dissolved in DMSO (9 mL) and solid was filtered off. The filtrate was purified using the Gilson (Basic, 50 x 250 mm column, 5 - 55% ACM/ 0.05% aqueous NH4OH, 16 min run). Fractions containing product were concentrated to give the title compound (219 g) as a solid. The solid was washed with hexanes then dissolved in DCM and purified using a Teledyne ISCG Combi-Flash system (liquid loading with DCM, 80G column, 0-2% MeQH/DCM/NhUOH, 10 min run). Fractions containing product were concentrated to give the title compound (682 mg; 43% yield) as a solid. *H NMR (400 MHz, CDCI3) 6 6.77 (t, J = 1.0 Hz, 1H), 4.45 (d, J = 1.0 Hz, 2H), 3.51 (s, 3H), 2.59 (d, J = 0 9 Hz, 3H), 2.49 (d, J = 1.0 Hz, 3H). ES-MS [M+l]+: 254. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science













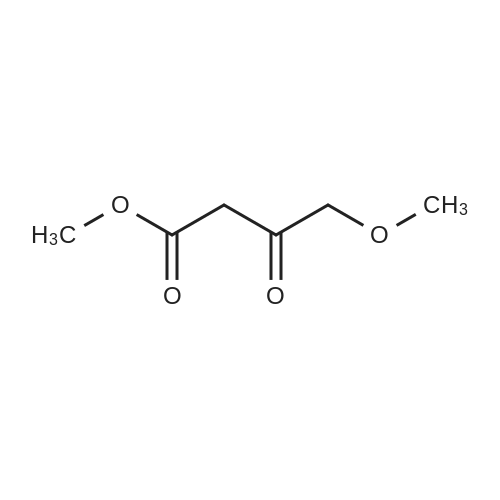

 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping