|
In dichloromethane; at 0℃; |
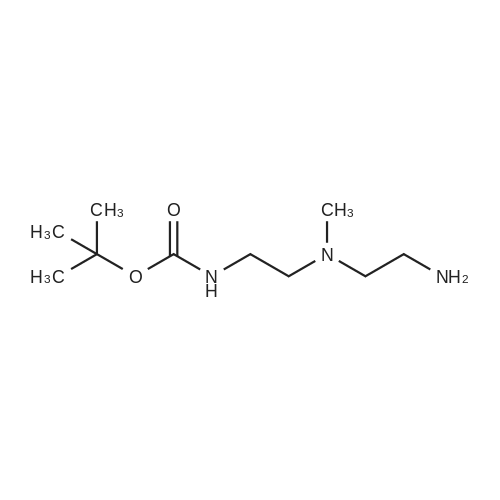
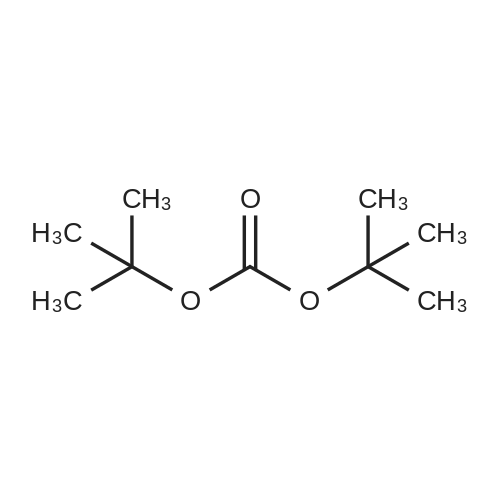
N1-(2-Aminoethyl)-N1-methylethane-1,2-diamine (5.0 g, 42.7 mmol) was dissolved in CH2Cl2 (100 mL) and cooled to 0 C. A solution of Boc2O (0.93 g, 4.27 mmol) in CH2 (10 mL) was then added dropwise at 0 C. over a period of 15 min. The resulting reaction mixture was stirred at 0 C. for 30 min and then warmed to room temperature. After stirring at room temperature for 2 h, the reaction mixture was diluted with CH2Cl2 (100 mL). The organic layer was washed with brine (3×25 mL), dried over Na2SO4, filtered and concentrated under reduced pressure to afford tert-butyl 2-((2-aminoethyl)(methyl)amino)ethylcarbamate (1.1 g).tert-Butyl 2-((2-aminoethyl)(methyl)amino)ethylcarbamate (400 mg, 1.84 mmol) was taken up in CH3CN (10 mL) along with nicotinic acid (227 mg, 1.84 mmol) and EDCI (353 mg, 2.02 mmol). The resulting reaction mixture was stirred at room temperature for 18 h and then diluted with EtOAc. The organic layer was washed with saturated aqueous NaHCO3, brine, dried over Na2SO4, filtered and concentrated under reduced pressure. The resulting residue was purified by silica gel chromatography (5% MeOH-CH2Cl2) to afford tert-butyl 2-(methyl(2-(nicotinamido)ethyl)amino)ethylcarbamate (180 mg, 30%). MS calculated for C16H26N4O3: 322.2; found: [M+H]+323.tert-Butyl 2-(methyl(2-(nicotinamido)ethyl)amino)ethylcarbamate (90 mg, 0.279 mmol) was taken up in a 25% TFA in CH2Cl2 solution (5 mL) and allowed to stand at room temperature for 3 h. The reaction mixture was concentrated under reduced pressure to afford the TFA salt of N-(2-((2-aminoethyl)(methyl)amino)ethyl)nicotinamide. This material was taken up in CH3CN (10 mL) along with (4Z,7Z,10Z,13Z,16Z,19Z)-docosa-4,7,10,13,16,19-hexaenoic acid (90 mg, 0.279 mmol), HATU (117 mg, 0.31 mmol) and DIEA (0.15 mL). The resulting reaction mixture was stirred at room temperature for 2 h. It was then diluted with EtOAc and washed successively with saturated aqueous NaHCO3 and brine. The organic layer was dried over Na2SO4, filtered and concentrated under reduced pressure. Purification by silica gel chromatography (5% MeOH-CH2Cl2) afforded N-(2-((2-(4Z,7Z,10Z,13Z,16Z,19Z)-docosa-4,7,10,13,16,19-hexaenamidoethyl)(methyl)amino)ethyl)nicotinamide (30 mg, 20%). MS calculated for C33H48N4O2: 532.38; found: [M+H]+533. |
|
In dichloromethane; at 0 - 20℃; for 2.75h; |
Example 8 Preparation of (4Z,7Z,10Z,13Z,16Z,19Z)-N-(2-((2-(2-(4-(4-chlorobenzoyl)phenoxy)-2-methylpropanamido)ethyl)(methyl)amino)ethyl)docosa-4,7,10,13,16,19-hexaenamide N1-(2-Aminoethyl)-N1-methylethane-1,2-diamine (5.0 g, 42.7 mmol) was dissolved in CH2Cl2 (100 mL) and cooled to 0 C. A solution of Boc2O (0.93 g, 4.27 mmol) in CH2Cl2 (10 mL) was then added dropwise at 0 C. over a period of 15 min. The resulting reaction mixture was stirred at 0 C. for 30 min and then warmed to room temperature. After stirring at room temperature for 2 h, the reaction mixture was diluted with CH2Cl2 (100 mL). The organic layer was washed with brine (3*25 mL), dried over Na2SO4, filtered and concentrated under reduced pressure to afford tert-butyl 2-((2-aminoethyl)(methyl)amino)ethylcarbamate (1.1 g). |
|
In dichloromethane; at 0 - 20℃; |
Example 8 Preparation of 5-((2-((2-((4Z,7Z,10Z,13Z,16Z,19Z)-docosa-4,7,10,13,16,19-hexaenamido)ethyl)(methyl)amino)ethyl)carbamoyl)-2-methylpyrazine 1-oxide (I-5) N1-(2-Aminoethyl)-N1-methylethane-1,2-diamine (5.0 g, 42.7 mmol) was dissolved in CH2Cl2 (100 mL) and cooled to 0 C. A solution of Boc2O (0.93 g, 4.27 mmol) in CH2Cl2 (10 mL) was then added dropwise at 0 C. over a period of 15 min. The resulting reaction mixture was stirred at 0 C. for 30 min and then warmed to room temperature. After stirring at room temperature for 2 h, the reaction mixture was diluted with CH2Cl2 (100 mL). The organic layer was washed with brine (3*25 mL), dried over Na2SO4, filtered and concentrated under reduced pressure to afford tert-butyl 2-((2-aminoethyl)(methyl)amino)ethylcarbamate (1.1 g). |
|
In dichloromethane; at 0 - 20℃; for 2.75h; |
Example 17 Preparation of (E)-methyl 4-(2-((2-(4Z,7Z,10Z,13Z,16Z,19Z)-docosa-4,7,10,13,16,19-hexaenamidoethyl)(methyl)amino)ethylamino)-4-oxobut-2-enoate (Compound I-4) N1-(2-Aminoethyl)-N-1-methylethane-1,2-diamine (5.0 g, 42.7 mmol) was dissolved in 100 mL of CH2Cl2 and cooled to 0 C. A solution of di-tert-butylcarbonate (0.93 g, 4.27 mmol) in CH2Cl2 (10 mL) was then added dropwise at 0 C. over a period of 15 min. The resulting reaction mixture was stirred at 0 C. for 30 min and then warmed to room temperature. After stirring at room temperature for 2 h, the reaction mixture was diluted with CH2Cl2 (100 mL). The organic layer was washed with brine (3*25 mL), dried (Na2SO4) and concentrated under reduced pressure to afford 1.1 g of tert-butyl 2-((2-aminoethyl)(methyl)amino)ethylcarbamate. |
|
In dichloromethane; at 0 - 20℃; for 2.75h; |
Example 7 Preparation of (S)-N-(2-((2-(5-(1,2-dithiolan-3-yl)pentanamido)ethyl)(methyl)amino)ethyl)-2-hydroxybenzamide (I-4) N-1-(2-Aminoethyl)-N1-methylethane-1,2-diamine (5.0 g, 42.7 mmol) was dissolved in CH2Cl2 (100 mL) and cooled to 0 C. A solution of di-tert-butylcarbonate (0.93 g, 4.27 mmol) in CH2Cl2 (10 mL) was then added dropwise at 0 C. over a period of 15 minutes. The resulting reaction mixture was stirred at 0 C. for 30 minutes and then warmed to room temperature. After stirring at room temperature for 2 h, the reaction mixture was diluted with CH2Cl2 (100 mL). The organic layer was washed with brine (3*25 mL), dried over Na2SO4 and concentrated under reduced pressure to afford 1.1 g of tert-butyl 2-((2-aminoethyl)(methyl)amino)ethylcarbamate. |
|
In dichloromethane; at 0 - 20℃; for 2.75h; |
Nl-(2-Aminoethyl)-Nl-methylethane-l,2-diamine (5.0 g, 42.7 mmol) was dissolved in 100 mL of CH2C12 and cooled to 0 C. A solution of di-tert-butylcarbonate (0.93 g, 4.27 mmol) in CH2C12 (10 mL) was then added dropwise at 0 C over a period of 15 min. The resulting reaction mixture was stirred at 0 C for 30 min and then warmed to room temperature. After stirring at room temperature for 2 h, the reaction mixture was diluted with CH2C12 (100 mL). The organic layer was washed with brine (3 x 25 mL), dried (Na2S04) and concentrated under reduced pressure to afford 1.1 g of tert-butyl 2-((2-aminoethyl)(methyl)amino)ethylcarbamate. |
|
In dichloromethane; at 0 - 20℃; |
Example 7; Preparation of (4Z,7Z,10Z,13Z,16Z,19Z)-N-(2-((2-((E)-6-(4-hydroxy-6-methoxy-7- methyl-3-oxo-1,3-dihydroisobenzofuran-5-yl)-4-methylhex-4- enamido)ethyl)(methyl)amino)ethyl)docosa-4,7,10,13,16,19-hexaenamide (I-3); N1-(2-Aminoethyl)-N1-methylethane-1,2-diamine (5.0 g, 42.7 mmol) was dissolved in 100 mL of CH2Cl2 and cooled to 0 C. A solution of di-tert-butylcarbonate (0.93 g, 4.27 mmol) in CH2Cl2 (10 mL) was then added dropwise at 0 C. over a period of 15 min The resulting reaction mixture was stirred at 0 C. for 30 min and then warmed to room temperature. After stirring at room temperature for 2 h, the reaction mixture was diluted with CH2Cl2 (100 mL). The organic layer was washed with brine (3×25 mL), dried (Na2SO4) and concentrated under reduced pressure to afford 1.1 g of tert-butyl 2-((2-aminoethyl)(methyl)amino)ethylcarbamate.tert-butyl 2-((2-aminoethyl)(methyl)amino)ethylcarbamate (200 mg, 0.922 mmol) was taken up in 5 mL of CH3CN along with mycophenolic acid (295 mg, 0.922 mmol) and EDC (194 mg, 1.01 mmol). The resulting reaction mixture was stirred at room temperature for 18 h and diluted with EtOAc. The organic layer was washed with brine, dried (Na2SO4) and concentrated under reduced pressure. Purification by chromatography (95% CH2Cl2, 5% MeOH) afforded 400 mg of (E)-tert-butyl 2-((2-(6-(4-hydroxy-6-methoxy- 7-methyl-3-oxo-1,3-dihydroisobenzofuran-5-yl)-4-methylhex-4- enamido)ethyl)(methyl)amino)ethylcarbamate (84% yield). This material was taken up in 10 mL of 4 M HCl in dioxane and allowed to stir at room temperature for 2 h. The mixture was diluted with EtOAc (30 mL) and concentrated under reduced pressure to afford the HC1 salt of (E)-N-(2-((2-aminoethyl)(methyl)amino)ethyl)-6-(4-hydroxy-6-methoxy-7-methyl-3-oxo- 1,3-dihydroisobenzofuran-5-yl)-4-methylhex-4-enamide. This material was then taken up in 10 mL of CH3CN along with (4Z,7Z,10Z,13Z,16Z,19Z)-docosa-4,7,10,13,16,19-hexaenoic acid (DHA, 252 mg, 0.77 mmol), HATU (322 mg, 0.85 mmol) and DIEA (540 3.1 mmol). The resulting reaction mixture was stirred at room temperature for 2 h and diluted with EtOAc (50 mL). The organic layer was washed with brine, dried (Na2SO4) and concentrated under reduced pressure. Purification by chromatography (95% CH2Cl2, 5% MeOH) afforded 300 mg of (4Z,7Z,10Z,13Z,16Z,19Z)-N-(2-((2-((E)-6-(4-hydroxy-6- methoxy-7-methyl-3-oxo-1,3-dihydroisobenzofuran-5-yl)-4-methylhex-4- enamido)ethyl)(methyl)amino)ethyl)docosa-4,7,10,13,16,19-hexaenamide (53% yield). MS (EI) calcd for C44H63N3O6: 729.47; found 730 (M+1). |
|
In dichloromethane; at 0 - 20℃; for 2.91667h; |
N1-(2-Aminoethyl)-N-1-methylethane-1,2-diamine (5.0 g, 42.7 mmol) is dissolved in CH2Cl2 (100 mL) and cooled to 0 C. A solution of Boc2O (0.93 g, 4.27 mmol) in CH2Cl2 (10 mL) is then added dropwise at 0 C. over a period of 15 min. The resulting reaction mixture is stirred at 0 C. for 30 min and then warmed to room temperature. After stirring at room temperature for 2 h, the reaction mixture is diluted with CH2Cl2 (100 mL). The organic layer is washed with brine (3*25 mL), dried over Na2SO4, filtered and concentrated under reduced pressure to afford tert-butyl 2-((2-aminoethyl)(methyl)amino)ethylcarbamate. |
|
In dichloromethane; at 0 - 20℃; for 2.75h; |
The same procedure outlined in example 8 was used, substituting tert-butyl2-((2-aminoethyl)(methyl)amino)ethylcarbamate for tert-butyl 2-aminoethylcarbamate. tert-Butyl 2-((2-aminoethyl)(methyl)amino)ethylcarbamate, in turn, could be prepared as follows: N1-(2-aminoethyl)-N1-methylethane-1,2-diamine (5.0 g, 42.7 mmol) was dissolved in 100 mL of CH2Cl2 and cooled to 0 C. A solution of di-tert-butylcarbonate (0.93 g, 4.27 mmol) in CH2Cl2 (10 mL) was then added dropwise at 0 C. over a period of 15 min. The resulting reaction mixture was stirred at 0 C. for 30 min and then warmed to room temperature. After stirring at room temperature for 2 h, the reaction mixture was diluted with CH2Cl2 (100 mL). The organic layer was washed with brine (3×25 mL), dried (Na2SO4) and concentrated under reduced pressure to afford 1.1 g of tert-butyl 2-((2-aminoethyl)(methyl)amino)ethylcarbamate. Compound I-31: MS (EI) calcd for C31H52N6O2 540.42; found 541 [M+H]+. |
| 1.1 g |
In dichloromethane; at 0 - 20℃; for 2.75h; |
[0244j Ni -(2-Aminoethyl)-N1 -methylethane- 1 ,2-diamine (5.0 g, 42.7 mmol) was dissolved in CH2C12 (100 mL) and cooled to 0 C. A solution of Boc2O (0.93 g, 4.27 mmol) in CH2C12 (10 mL) was then added dropwise at 0 C over a period of 15 mm. The resulting reaction mixture was stirred at 0 C for 30 mm and then warmed to room temperature. After stirring at room temperature for 2 h, the reaction mixture was diluted with CH2C12 (100 mL). The organic layer was washed with brine (3 x 25 mL), dried over Na2SO4, filtered andconcentrated under reduced pressure to afford tert-butyl 2-((2-aminoethyl)(methyl)amino)ethylcarbamate (1.1 g). |
| 1.1 g |
In dichloromethane; at 0 - 20℃; for 0.0458333h; |
Example 10 Preparation of V-(2-((2-(4Z,7Z,10Z,13Z,16Z,19Z)-docosa-4,7,10,13,16,19- hexaenamidoethyl)(methyl)amino)ethyl)nicotinamide (1-2) [0332] M-(2-Aminoethyl)-M-methylethane-l,2-diamine (5.0 g, 42.7 mmol) was dissolved in CH2C12 (100 mL) and cooled to 0 C. A solution of Boc20 (0.93 g, 4.27 mmol) in CH2C12 (10 mL) was then added dropwise at 0 C over a period of 15 min. The resulting reaction mixture was stirred at 0 C for 30 min and then warmed to room temperature. After stirring at room temperature for 2 h, the reaction mixture was diluted with CH2CI2 (100 mL). The organic layer was washed with brine (3 x 25 mL), dried over Na2S04, filtered and concentrated under reduced pressure to afford tert-butyl 2-((2- aminoethyl)(methyl)amino)ethylcarbamate (1.1 g). |
| 1.1 g |
In dichloromethane; at 0 - 20℃; for 2.75h; |
Example 7 Preparation of (4Z,7Z,10Z,13Z,16Z,19Z)-N-(2-((2-(2-(1-(4-chlorobenzoyl)-5-methoxy-2-methyl-1H-indol-3-yl)acetamido)ethyl)(methyl)amino)ethyl)docosa-4,7,10,13,16,19-hexaenamide (I-14) N1-(2-Aminoethyl)-N1-methylethane-1,2-diamine (5.0 g, 42.7 mmol) was dissolved in 100 mL of CH2Cl2 and cooled to 0 C. A solution of di-tert-butylcarbonate (0.93 g, 4.27 mmol) in CH2Cl2 (10 mL) was then added dropwise at 0 C. over a period of 15 mm. The resulting reaction mixture was stirred at 0 C. for 30 min and then warmed to room temperature. After stirring at room temperature for 2 h, the reaction mixture was diluted with CH2Cl2 (100 mL). The organic layer was washed with brine (3×25 mL), dried (Na2SO4) and concentrated under reduced pressure to afford 1.1 g of tert-butyl 2-((2-aminoethyl)(methyl)amino)ethylcarbamate. (0377) tert-butyl 2-((2-aminoethyl)(methyl)amino)ethylcarbamate (150 mg, 0.69 mmol) was taken up in 10 mL of CH3CN along with indomethacin (247 mg, 0.69 mmol) and EDC (146 mg, 0.76 mmol). The resulting reaction mixture was stirred at room temperature for 2 h and then diluted with EtOAc (40 mL). The organic layer was washed with brine, dried (Na2SO4) and concentrated under reduced pressure. Purification by chromatography (95% CH2Cl2, 5% MeOH) afforded 360. Mg of the Boc-protected intermediate (93% yield). This material was taken up in 10 mL of 4 M HCl in dioxane and allowed to stir at room temperature for 10 min. The reaction mixture was concentrated under reduced pressure to afford the HCl salt of N-(2-((2-aminoethyl)(methyl)amino)ethyl)-2-(1-(4-chlorobenzoyl)-5-methoxy-2-methyl-1H-indol-3-yl)acetamide. (0378) This HCl salt of N-(2-((2-aminoethyl)(methyl)amino)ethyl)-2-(1-(4-chlorobenzoyl)-5-methoxy-2-methyl-1H-indol-3-yl)acetamide (0.38 mmol) was taken up in 5 mL of CH3CN along with DHA (210 mg, 0.64 mmol), HATU (267 mg, 0.67 mmol) and DMA (334 μL, 2.01 mmol). The resulting reaction mixture was stirred at room temperature for 2 h and diluted with EtOAc (25 mL). The organic layer was washed with brine, dried (Na2SO4) and concentrated under reduced pressure. Purification by chromatography (95% CH2Cl2, 5% MeOH) afforded 320 mg of (4Z,7Z,10Z,13Z,16Z,19Z)-N-(2-((2-(2-(1-(4-chlorobenzoyl)-5-methoxy-2-methyl-1H-indol-3-yl)acetamido)ethyl)(methyl)amino)ethyl)docosa-4,7,10,13,16,19-hexaenamide (65% yield). MS (EI) calcd for C46H59ClN4O4: 766.42; found 767 (M+1). |
| 8.2 g |
|
Procedure: 2,2′-Diamino N-methyldiethylamine 12 (10.51 g, 89.6 mmoles) was dissolved in methanol (100 ml). The solution was cooled to 0 C. and trifluoroacetic acid (10.21 g) in methanol (30 ml) was added dropwise in 1 hour, 50 ml of water was then added, and the mixture stirred for 1 hour. BOC2O (19.5 g, 89.6 mmoles) and iodine (2.25 g, 8.96 mmoles) in ethanol (75 ml) was added dropwise over 1 hour and then the mixture stirred for 5 hours. The reaction was neutralized with sodium hydroxide solution, concentrated, and the crude partitioned between dichloromethane and water (100 ml/25 ml). The organic layer was separated, the aqueous layer extracted 2× dichloromethane (50 ml), the organic extracts combined, washed with 10% sodium bisulfite solution, and dried over MgSO4. Filtration, and concentration gave crude product which was purified by flash chromatography using dichloromethane/methano/aq NH3 90:10:2 and gave 8.2 grams of white solid 13. 1H NMR (400 MHz, CDCl3): δ 5.04 (1H, hr s), 3.18 (2H, q), 2.70 (2H, t), 2.39 (4H, m), 2.18 (3H, s), 1.77 (2H, br s), 1.40 (9H, s). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science













 HazMat Fee +
HazMat Fee +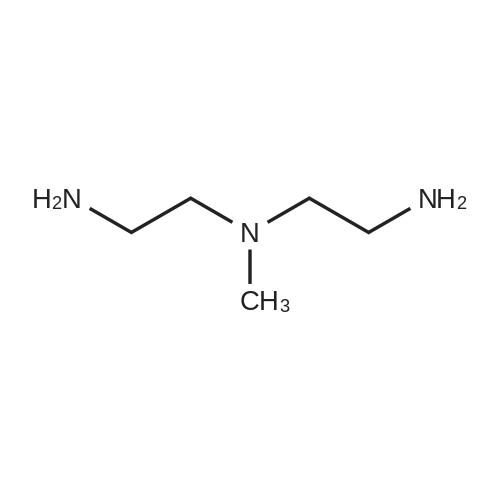

 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping