|
|
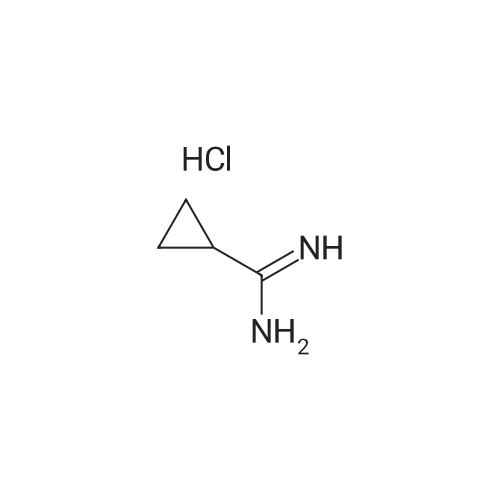
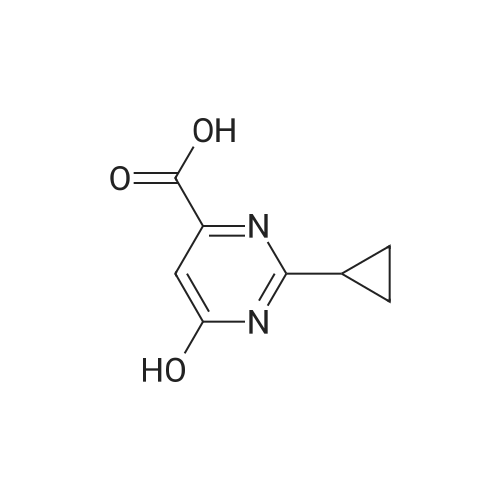
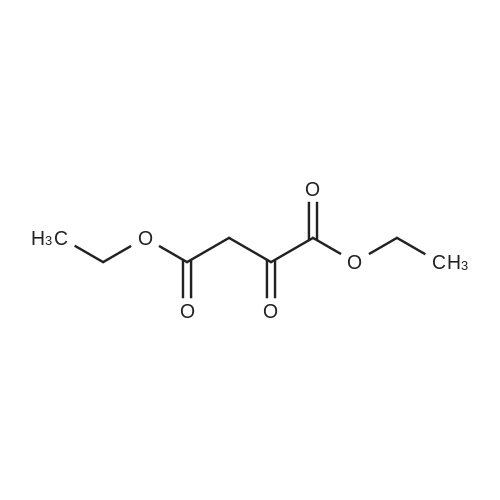
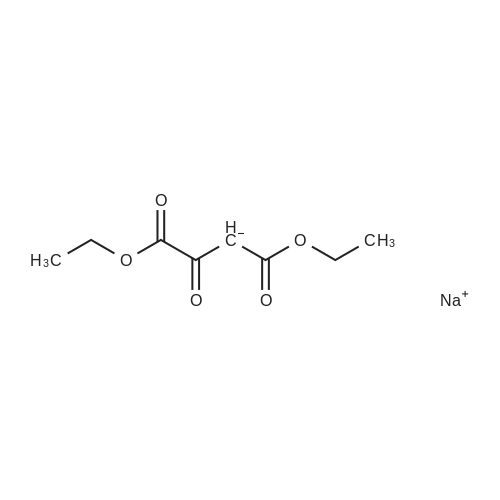
EXAMPLE 2 Preparation of 6-amino-5-chloro-2-cyclopropyl-4-pyrimidinecarboxylic acid (Compound 135) Step Al : Preparation of 2-cyclopropyl-1, 6-dihydro-6-oxo-4-pyrimidinecarboxylic acid To a mixture of diethyl oxalacetate sodium salt (150 g, 714 mmol) in methanol (300 mL) and water (150 mL) warmed to 30 °C was added 50percent aqueous sodium hydroxide (56 g, 700 mmol) in water (60 mL) over 30 minutes, over which time the temperature remained at 25-30 °C and the pH at 11-12. Then the stirred mixture was heated for an additional 30 min at 35 °C. To this mixture was added <strong>[57297-29-7]cyclopropanecarboximidamide monohydrochloride</strong> (64 g, 530 mol) in portions over 15 minutes. The orange solution was heated to 50 °C over 30 minutes and held at that temperature for 3 h. The reaction mixture was cooled to 35 °C, and concentrated hydrochloric acid (ca. 70 g, 0.7 mol) was added gradually (resulting in foaming) over 30 minutes at 30-40 °C until the pH was about 1.5- 2.5. The mixture was concentrated with a rotary evaporator at 35-40 °C to remove alcohols, stirred for 3-4 h at 25 °C to complete crystallization of the product. After the mixture was cooled to 0 °C the solid was collected by filtration. The solid was washed with water (2 x 60 mL), suction-dried, and then dried in a vacuum-oven at 60 °C to afford the title compound as a beige solid (ca. 60 g). 1H NMR (DMSO-d6) otilde; 6. 58 (s, 1H), 1.95 (m, 1H), 1.0 (m, 4H).; Step A2: Another preparation of 2-cyclopropyl-1,6-dihydro-6-oxo-4-pyrimidine- carboxylic acid To a mixture of diethyl oxalacetate sodium salt (210 g, 950 mmol) in methanol (500 mL) and water (400 mL) was added 50percent aqueous sodium hydroxide (80 g, 1.0 mol) in water (60 mL) over 30 minutes, over which time the temperature remained at 25-30 °C and the pH at 11-12. Then the stirred mixture was heated for an additional 30 min at 30 °C. To this mixture was added <strong>[57297-29-7]cyclopropanecarboximidamide monohydrochloride</strong> (110 g, 910 mol). The orange solution was heated to 50 °C over 30 minutes and held at that temperature for 5 h. The reaction mixture was cooled to 30 °C and concentrated to half volume at reduced pressure at 35-40 °C and concentrated hydrochloric acid (140 g, 1.4 mol) was added gradually (resulting in foaming) over 30 minutes at 25-30 °C until the pH was about 1-2. The mixture was stirred at 5 °C for 1 h to complete crystallization of the product. After the mixture was cooled to 0 °C the solid was collected by filtration. The solid was washed with water (3 x 60 mL), suction-dried, and then dried in a vacuum-oven at 70 °C to afford the title compound as a beige solid (100 g) ; m. p. 235-236 °C (dec. ). 1H NMR (DMSO-d6) 8 6.58 (s, 1H), 1.95 (m, 1H), 1.0 (m, 4H). |
| 60 g |
|
To a mixture of diethyl oxalacetate sodium salt (150 g, 714 mmol) in methanol (300 mL) and water (150 mL) warmed to 30° C. was added 50percent aqueous sodium hydroxide (56 g, 700 mmol) in water (60 mL) over 30 minutes, over which time the temperature remained at 25-30° C. and the pH at 11-12. Then the stirred mixture was heated for an additional 30 min at 35° C. To this mixture was added <strong>[57297-29-7]cyclopropanecarboximidamide monohydrochloride</strong> (64 g, 530 mol) in portions over 15 minutes. The orange solution was heated to 50° C. over 30 minutes and held at that temperature for 3 h. The reaction mixture was cooled to 35° C., and concentrated hydrochloric acid (ca. 70 g, 0.7 mol) was added gradually (resulting in foaming) over 30 minutes at 30-40° C. until the pH was about 1.5-2.5. The mixture was concentrated with a rotary evaporator at 35-40° C. to remove alcohols, stirred for 3-4 h at 25° C. to complete crystallization of the product. After the mixture was cooled to 0° C. the solid was collected by filtration. The solid was washed with water (2*60 mL), suction-dried, and then dried in a vacuum-oven at 60° C. to afford the title compound as a beige solid (ca. 60 g). 1H NMR (DMSO-d6) delta 6.58 (s, 1H), 1.95 (m, 1H), 1.0 (m, 4H). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping