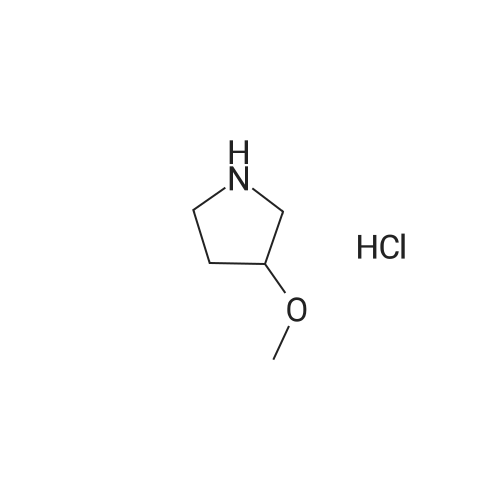
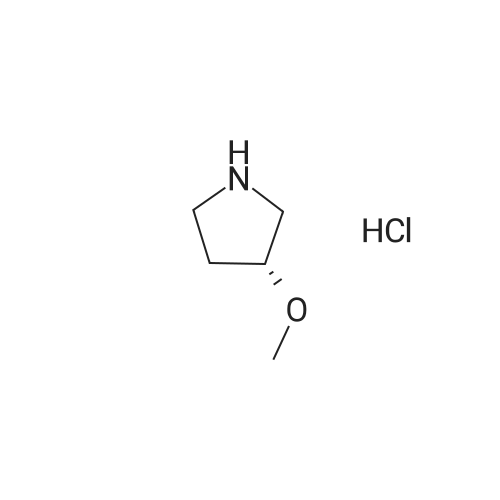
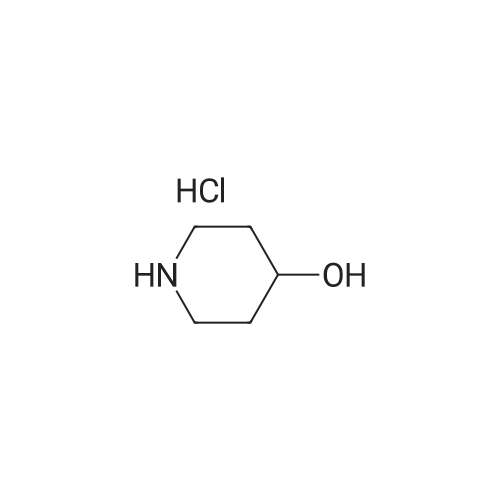
|
With hydrogenchloride; In ethyl acetate; at 0 - 20℃; for 13h; |
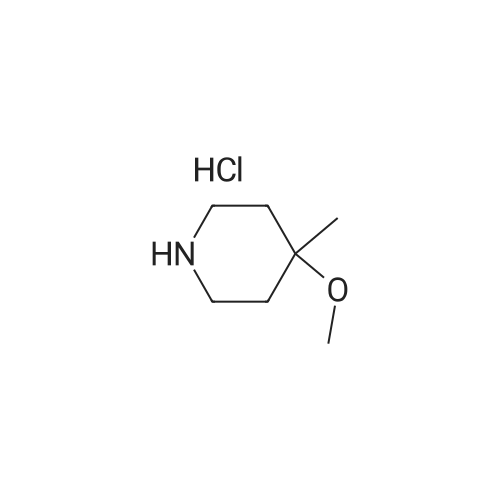
Ethyl acetate (200 mL) was added to the residue, and the mixture was cooled to 0 C. and stirred. A 4N solution of hydrogen chloride in ethyl acetate (100 mL) was then gradually added over 10 minutes, and the temperature was slowly raised to room temperature. After stirring for 13 hours, the reaction mixture was concentrated under reduced pressure. The residue was dissolved in a small amount of dichloromethane. An excess of ethyl acetate was then added and the precipitated solid was filtered out and dried under reduced pressure to give 17.0 g of the title compound as colorless crystals. 1H-NMR (400 MHz, CDCl3) delta: 1.95-2.02 (m, 2H), 2.05-2.15 (m, 2H), 3.14-3.30 (m, 4H), 3.32 (s, 3H), 3.52-3.57 (m, 1H). The 1H of NH could not be identified. |
|
With hydrogenchloride; In methanol; |
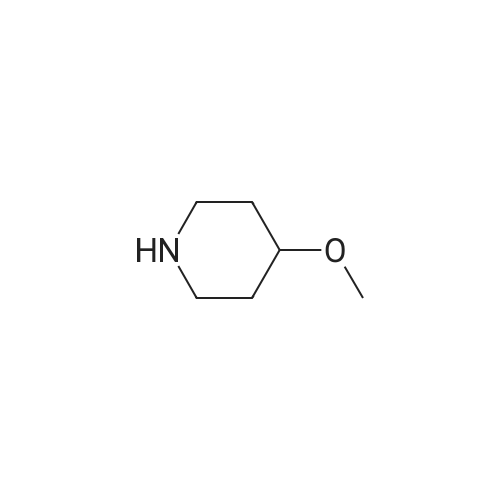
This compound was cooled at 0 C. and 30 mL of 3N hydrochloric acid in methanol was added. After stirring for 19 hours at room temperature the reaction mixture was concentrated to give 2.4 g of 4-methoxypiperidine hydrochloride. 1H-NMR 200 MHz (CD3OD) delta: 1.76-2.12 (4H, m), 3.03-3.61 (5H, m), 3.36 (3H, s). |
|
With hydrogenchloride; In 1,4-dioxane; at 20℃; for 1h; |
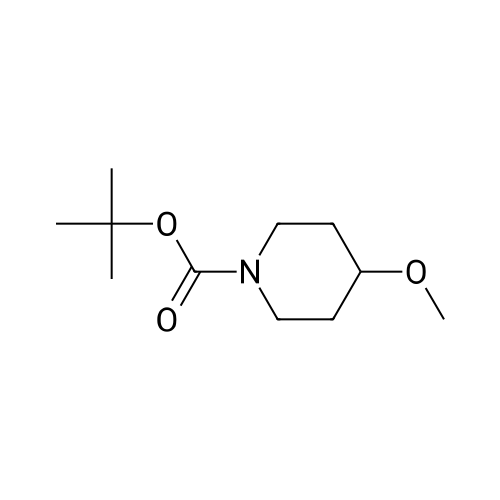
4N HCl-Dioxane (10 mL) was added to a solution of the above 4-methoxypiperidine-1-carboxylic acid tert-butyl ester (5.34 g) in 1,4-dioxane (10 mL) at room temperature, and the mixture was stirred for 30 minutes. 4N HCl-Dioxane (20 mL) was further added thereto, and the resultant mixture was stirred for 30 minutes. The reaction solvent was removed under reduced pressure, and the obtained solid was washed with ethyl acetate, to thereby give the title compound (3.55 g) . 1H-NMR (400MHz, DMSO-d6) delta: 1.68 (2H,m), 1.93 (2H,m), 2.91 (2H, m), 3.08(2H,m), 3.23(3H,s), 3.42 (1H, q, J=3. 90Hz). |
|
With hydrogenchloride; In 1,4-dioxane; at 20℃; for 1h; |
[Referential Example 27] 4-Methoxypiperidine hydrochloride 4-Methoxypiperidine-1-carboxylic acid tert-butyl ester (5.34 g) obtained in step 1) of Referential Example 11 was dissolved in 1,4-dioxane (10 mL), and 4N HCl-dioxane (10 mL) was added to the solution at room temperature, followed by stirring for 30 minutes. 4N HC1-dioxane (20 mL) was added to the reaction mixture, and the resultant mixture was stirred for 30 minutes. The reaction solvent was evaporated under reduced pressure, and the resultant solid was collected through filtration by use of ethyl acetate, to thereby give the title compound (3.55 g). 1H-NMR(400MHz,DMSO-d6)delta:1.68(2H,m), 1.93(2H,m), 2.91(2H,m), 3.08(2H,m), 3.23(3H,s), 3.42(1H,q,J=3.90Hz). |
|
With hydrogenchloride; In 1,4-dioxane; at 20℃; for 1h; |
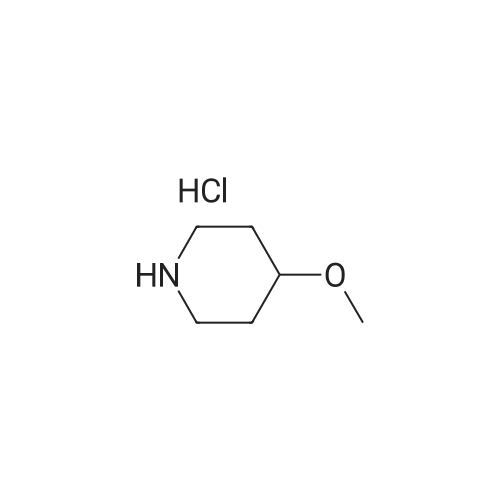
[Reference Example 23] 4-Methoxypiperidine hydrochloride [Show Image] A 4 N hydrochloric acid-dioxane solution (10 mL) was added to a solution of 4-methoxypiperidine-1-carboxylic acid tert-butyl ester (5.34 g) of Reference Example 17-(1) in 1, 4-dioxane (10 mL) at room temperature, and the resultant mixture was stirred for 30 minutes. Further, a 4 N hydrochloric acid-dioxane solution (20 mL) was added to the mixture, which was then stirred for 30 minutes. The reaction solvent was evaporated under reduced pressure, and the solid thus obtained was filtered with ethyl acetate, to obtain the title compound (3.55 g). 1H-NMR(400MHz, DMSO-d6)delta: 1.68(2H, m), 1.93(2H, m), 2.91(2H, m), 3.08(2H, m), 3.23(3H, s), 3.42(1H, q, J=3.90Hz). |
|
With hydrogenchloride; In 1,4-dioxane; at 20℃; for 0.5h; |
2) Title compound A 4 mol/L hydrochloric acid-dioxane solution (10 mL) was added to a solution of the 4-methoxypiperidine-1-carboxylic acid tert-butyl ester (5.34 g) thus obtained in 1,4-dioxane (10 mL) at room temperature, and the resultant mixture was stirred for 30 minutes. A 4 mol/L hydrochloric acid-dioxane solution (20 mL) was further added thereto, and the mixture was stirred for 30 minutes. The reaction solvent was evaporated under reduced pressure, and the solid thus obtained was washed with ethyl acetate, to obtain the title compound (3.55 g). 1H-NMR (400MHz, DMSO-d6) delta: 1.68 (2H, m), 1.93 (2H, m), 2.91 (2H, m), 3.08 (2H, m), 3.23 (3H, s), 3.42 (1H, q, J=3.90Hz). |
|
With hydrogenchloride; In 1,4-dioxane; methanol; at 20℃; for 3h; |
To a solution OF 4-METHOXYPIPERIDINE-1-CARBOXYLIC acid tert-butyl ester (Preparation 67, 1. 58G, 7. 34MMOL) in methanol (20ML) was added hydrochloric acid in 1,4-dioxane (4M, lOmL) and the mixture stirred for 3H at rt. Concentration III VACUO gave an oil which was redissolved in water (LOOML). The aqueous layer was washed with ethyl acetate (2X30ML) and concentrated to give the title compound as colourless solid. ON (D2O): 1.80, 2.14 (4H, 2m), 3.13 (2H, m), 3.38 (2H, m), 3.40 (3H, s), 3.68 (m, 1H). |
|
With hydrogenchloride; In 1,4-dioxane; at 20℃; for 96h; |
4-Methoxypiperidine (286). A mixture of terf-butyl 4-hydroxy-1-piperidinecarboxylate (284) (Dailewicz, J. C; et al., J. Med. Chem. 2002, 45, 2432-2453) (19.7 g, 98 mmol), crushed KOH (11.0 g, 196 mmol) and MeI (7.3 mL, 118 mmol) in DMSO (100 mL) was stirred at20 0C for 16 h under N2. The mixture was poured into water (500 mL) and extracted with Et2O (2 x 150 mL). The combined organic fraction was washed with water (2 x 50 mL), dried and the solvent evaporated to give methyl ether 285 (19.1 g, 91%) as a white solid: 1H NMR delta 3.71-3.78 (m, 2 H, CH2N), 3.31-3.39 (m, 4 H, CHO, OCH3), 3.06-3.12 (m, 2 H, CH2N), 1.80-1.85 (m, 2 H, CH2), 1.45-1.54 (m, 2 H, CH2), 1.43 [s, 9 H, C(CH3)3]. A solution of HCI in dioxane (4 M, 67 mL, 266 mmol) was added to a stirred solution of methyl ether 285 (19.1 g, 88.7 mmol) in dioxane (100 mL) and the mixture stirred at 20 0C for 96 h.The solvent was evaporated and the residue dried to give the amine hydrochoride 286 as a white solid: 1H NMR [(CD3)2SO] delta 8.99 (br s, 2 H, NH.HCI), 3.40-3.46 (m, 1 H, CHO), 3.25 (s, 3 H, OCH3), 3.07-3.12 (m, 2 H, CH2N)1 2.88-2.94 (m, 2 H, CH2N), 1.91- 1.99 (m, 2 H, CH2), 1.63-1.74 (m, 2 H, CH2). The hydrochloride was dissolved in water (50 mL), the pH adjusted to 10 with CNH3 and the mixture extracted with CHCI3 (4 x 50 mL) to give the free base, which was used without further purification. |
| 1.42 g |
With hydrogenchloride; In 1,4-dioxane; dichloromethane; at 20℃; for 5h;Cooling with ice; |
The compound 486 tert-butyl 4-hydroxypiperidin-1-carboxylate (2.01 g, 10.0 mmol) was dissolved in 22 THF (10 mL), cooled in ice-water bath and 473 NaH (60%, 288 mg, 12.0 mmol) was added in portions, and stirred in the ice-water bath for 30 minutes. 154 Methyl iodide (0.65 mL, 10.5 mmol) was added in one portion, and the mixture was stirred at room temperature overnight. The reaction mixture was concentrated, 10 mL EA and 10 mL brine were added, the layers were separated, the water phase was extracted with ethyl acetate (10 mL×3), the organic phase was combined and washed with brine, dried over anhydrous sodium sulfate, filtered and concentrated to afford a colorless oily liquid (2.12 g). The oily liquid obtained above (2.12 g) was dissolved in 402 DCM (10 mL), 4M 51 HCl in 113 dioxane (10 mL) was added in ice-water bath, and stirred at room temperature for 5 hours. The reaction mixture was concentrated to afford a white 492 solid (1.42 g). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping