| 98% |
With potassium carbonate; In N,N-dimethyl-formamide; at 20℃;Inert atmosphere; |
General procedure: To a suspension ofphenol (16, 17, 22, and 23) (1 mmol, 1.0 equiv) and K2CO3(3 mmol, 3 equiv) in anhydrous DMF(5 mL) was added allylbromide (1.5 mmol, 1.5 equiv) dropwise under nitrogenatmosphere at room temperature. The mixture was stirred atroom temperature for 1 h. After completion of the reaction,water (10 mL) was added. The mixture was then extractedwith ether (2 × 30 mL), the combined organic layer waswashed with brine (2 × 30 mL), dried over anhydrousNa2SO4, filtered and the filtrate was concentrated in vacuo.The crude residue was purified by column chromatography (EtOAc/hexane = 1/8) to yield the allyl protected aldehyde(11, 18, 7, and 9). |
| 98% |
With potassium carbonate; In N,N-dimethyl-formamide; at 20℃; for 1h;Inert atmosphere; |
Phenol (16, 17, 22 and 23) (1 mmol, 1.0 eq.) And Allyl bromide (1.5 mmol, 1.5 eq.) Was added dropwise to a suspension of K2CO3 (3 mmol, 3 eq.) In anhydrous DMF (5 mL) under nitrogen atmosphere at room temperature. The mixture was stirred at room temperature for one hour. After completion of the reaction, water (10 ml) was added. The mixture was extracted with ether (2 x 30 mL) and the organic layer was washed with brine (2 x 30 mL), dried over anhydrous Na2SO4, filtered and the filtrate was concentrated in vacuo. The crude compound was purified by column chromatography (EtOAc / hexane = 1/8) to give aldehydes 11, 18, 7 and 9 protected with allyl groups. |
| 98% |
With 18-crown-6 ether; potassium carbonate; potassium iodide; In acetonitrile; at 75℃; for 18h; |
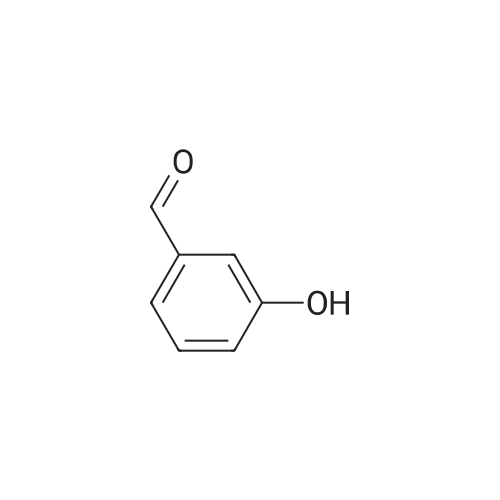
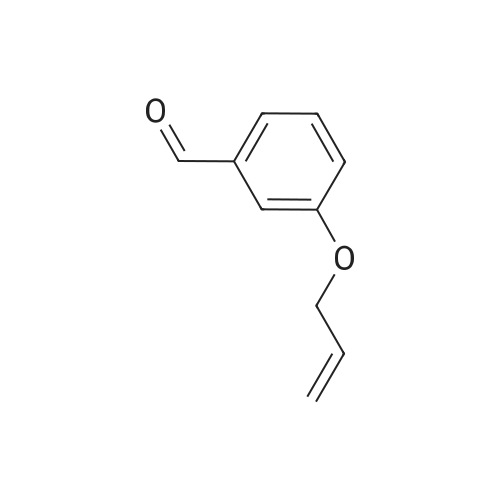
To a stirred solution of 3-hydroxybenzaldehyde (8.0 g, 65.6 mmol) in acetonitrile (80 mL) was added allylbromide (11.0 mL, 131 mmol), KI (1.09 g, 6.56 mmol), 18-crown-6 (864 mg, 3.26 mmol) and K2CO3 (26.4 g, 191 mmol) at rt, after which the solution was refluxed at 75 C for 18 h. The K2CO3 was filtered off and the solvent was removed under reduced pressure·H2O (100 mL) was added and the aqueous layer extracted with EtOAc (2 * 100 mL). The organic layer was separated, washed with brine (50 mL), dried (Na2SO4) and the solvent removed under reduced pressure to yield the title compound as an orange oil, which was used without further purification (10.5 g, 64.4 mmol, 98%). Rf = 0.38 (5% EtOAc in hexane). IR: lambdamax = 2861 (w, C-H), 1681 (s, C=O), 1598 (s, C=C), 1483 (m, C=C), 1457 (m, C=C). 1H NMR (400 MHz, CDCl3): deltaH = 9.92 (1H, s, H10), 7.37-7.44 (2H, m, H6 & H7), 7.36 (1H, dd, J = 2.0, 1.0 Hz, H9), 7.15 (1H, dt, J = 7.2, 2.4 Hz, H5), 5.96-6.09 (1H, ddt, J = 17.2, 10.5, 5.2 Hz, H2), 5.40 (1H, dq, J = 17.3, 1.5 Hz, H1t), 5.28 (1H, dq, J = 10.6, 1.4 Hz, H1c), 4.55 (2H, dt, J = 5.3, 1.4 Hz, H3). 13C NMR (101 MHz, CDCl3): deltaC = 192.0 (C10), 159.1 (C4), 137.8 (C8), 132.7 (C2), 130.1 (C6), 123.5 (C7), 122.0 (C5), 118.0 (C1), 113.1 (C9), 68.9 (C3) . HRMS (ESI+): m/z found [M+H]+ 163.0751, C10H11O2 required 163.0754. |
| 94.8% |
With potassium carbonate; In N,N-dimethyl-formamide; at 25 - 45℃; |
Meta hydroxy benzaldehyde (500 g) and potassium carbonate (679 g) were added to DMF (1000 ml). Allyl bromide (569.0 g) was gradually added to the reaction mixture, which was stirred at 25-45C. (0113) After completion of the reaction, water was added to the reaction mass followed by extraction with methyl tertiary butyl ether (MTBE). The organic layer was separated, washed with 1% sodium hydroxide solution (49 g NaOH in 490 ml water) to give compound (2). Yield: 630 g (94.8 %) |
| 92% |
With sodium perborate; In water; at 20℃; for 8.5h;Green chemistry; |
General procedure: To the 25 mL round bottomed flask containing 5 mL of water, were added beta-Naphthol (1d, 0.50 g, 1 eq.), sodium perborate (3, 0.214 g, 0.4 eq.), and allyl bromide (2, 0.462 g, 1.1 eq.) and reaction mixture was stirred at room temperature as given time. Progress of the reaction was monitored by TLC. After completion of the reaction (6h), the product was extracted with ethyl acetate (3x15 mL) and combined organic layers were dried over anhydrous sodium sulfate and concentrated under reduced pressure. The crude residue thus obtained was purified by silica gel column chromatography (100-200 mesh) to afford pure compound 4d. Similar procedure was adopted for the compounds 4a-c, and 4e-j. |
| 89% |
With caesium carbonate; In N,N-dimethyl acetamide; ethyl acetate; |
EXAMPLE 58A 3-(allyloxy)benzaldehyde 3-Hydroxybenzaldehyde (1.00 g, 8.19 mmol) and allyl bromide (0.780 mL, 9.01 mmol) in N,N-dimethylacetamide (35 mL) was treated with Cs2CO3 (4.01 g, 12.3 mmol) and allowed to stir at room temperature for 12 hours. The reaction mixture was diluted with diethyl ether (150 mL), washed with 1N HCl, saturated NaHCO3, brine, dried (MgSO4), filtered and the filtrate concentrated. The residue was purified by flash chromatography (silica gel, using 7% ethyl acetate/hexanes as eluent) to provide the title compound as a clear oil (1.19 g, 89%). 1H NMR (CDCl3, 300 MHz) delta 4.82 (m, 2H), 5.25-5.5.50 (m, 2H), 6.03 (m, 1H), 7.18-7.52 (m, 4H), 9.95 (s, 1H); MS (DCI+) 163 (M+H)+. |
| 83% |
With potassium carbonate; In N,N-dimethyl-formamide; at 20℃; for 3h; |
General procedure: To a solution of 3-hydroxybenzaldehyde 1a (81.9 mmol, 10.0 g) and K2CO3 (1.5 g) in DMF (100.0 mL) was added allyl bromide (7.8 mL) and the mixture was stirred for 3 h at rt. The reaction mixture was quenched with water (50 mL) and the organic phase was extracted into Et2O (3×100 mL), dried over Na2SO4 and the solvent removed at reduced pressure to give a yellow liquid 2a (11.0 g, 83%): FTIR (KBr) 3071, 2818, 1697 cm-1; 1H NMR (200 MHz): delta 9.91 (s, 1H), 7.41 (m, 3H), 7.17 (m, 1H), 6.04 (m, 1H), 5.41 (dd, J 16.0, 6.0 Hz, 1H), 5.30 (dd, J 10.0, 6.0 Hz, 1H), 4.58 (d, J 5.6 Hz, 2H); 13C NMR (50 MHz, CDCl3): delta 191.7, 159.1, 137.7, 132.8, 130.1, 123.6, 122.1, 118.0, 113.1, 68.9; MS(EI) m/z: 162 (M+, 100), 147 (47), 121 (94), 65 (81). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping