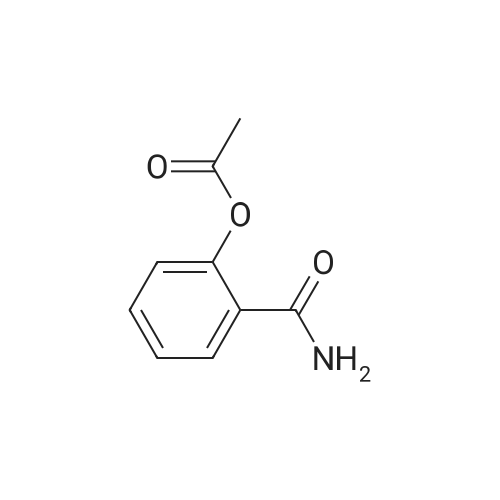
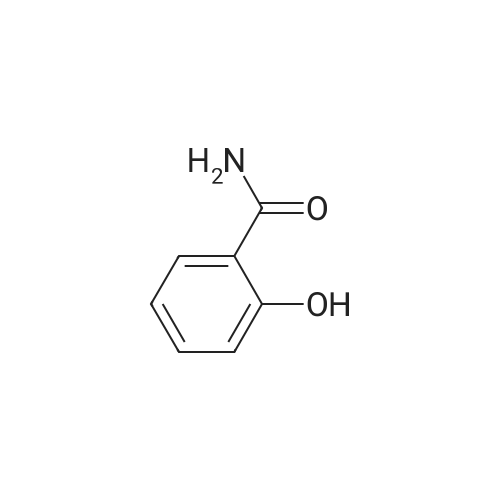
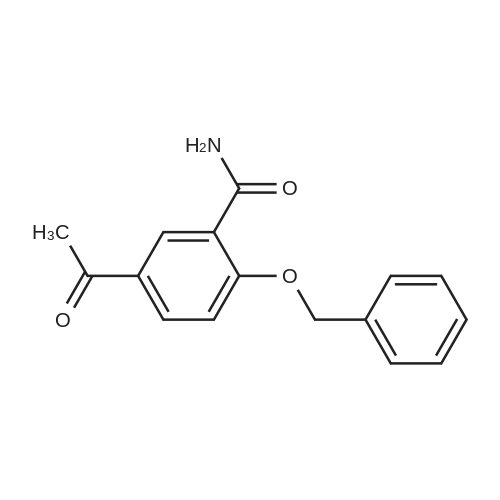
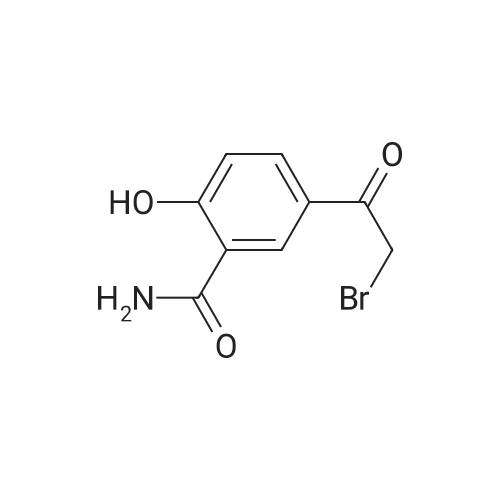
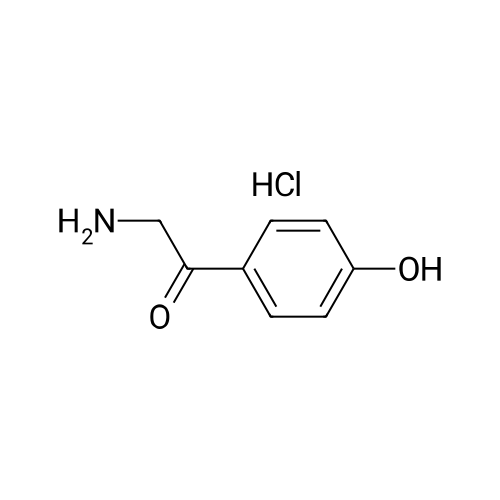
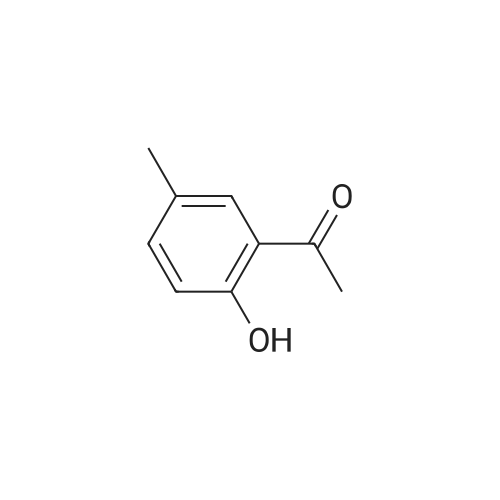
| 92.2% |
With aluminum (III) chloride; sodium chloride; at 140℃; for 0.666667h; |
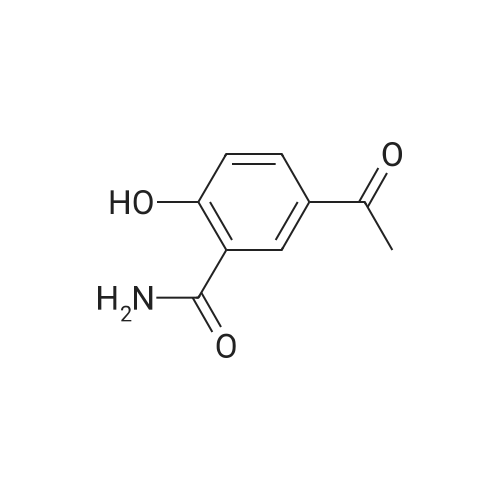
1), NaCl-AlCl3 low melting point mixed molten salt system preparation:A 100 ml three-necked flask,Mechanical agitation,Condensate tube (linked to tail gas HCl absorption device),0 ~ 200 thermometer and constant pressure dropping funnel consisting of the device placed in a constant temperature oil bath;Quickly weighed 0.0648 mol (about 8.64 g) of anhydrous aluminum chloride,0.0648 mol (about 3.79 g) of sodium chloride was added to the flask,Open the mechanical stirring, heating to 140 ,The solid is melted and the temperature is stable.Note: After about 25 minutes, anhydrous aluminum chloride, sodium chloride are in a molten state.2), weigh 0.036 mol (about 5.00 g) of salicylamide under stirring to add the flask(Flask temperature 140 )And melted,The temperature inside the flask is stable.3), weighed 0.0432 mol (about 3.39 g) of acetyl chloride,With a constant pressure dropping funnel through the condenser tube into the system,About 10min drops finished.The temperature of the drop was maintained at 140 C for 0.5 h,The reaction is complete.which is,The reaction was carried out in a NaCl-AlCl3 low melting point mixed molten salt system as a solvent.4), immediately after the completion of the reaction within the system slowly (that is, even within 5 minutes evenly added)60ml acid (composed of 1ml concentrated hydrochloric acid and 59ml ice water mixture)Producing a pale yellow solid,After the addition of the acid solution, the stirring was continued for 30 min at room temperature.At this point no longer produce light yellow solid;Get the suspension.5), filter the suspension,To give a pale yellow solid,And washed three times with hot water at 80 C (5 ml each)Drying (80 C, drying for 5 h) yields the crude product.6), and the resulting crude product was added with 20 ml of ethanol,Heated to reflux temperature,The crude product is completely dissolved,After the re-crystallization in the ice bath,Precipitation of the crystal filter drying (80 , drying 5h),To give 5-acetylsalicylamide as a white solid(Purity ? 98.1%), the yield was 92.2%. |
| 88.5% |
|
Step 3, 15g of AlCl3 and 7g of anhydrous, and 0.5g of self-made modified nano-scale solid acid catalyst was added to the vessel, stirred and heated to 180 C, then the intermediate salicylamide obtained above was added, and magnetic stirring was continued for 3 h;Step 4, then add 15g of acetyl chloride at a rate of 60 drops per minute, magnetically stirred, oil bath 180 C 4h;Step 5, adding 100 ml of 5% (V) hydrochloric acid solution to the reaction system, magnetic stirring at 60 C for 2 h, filtering, while recovering the self-made modified nano-scale solid acid catalyst, the filtrate is washed 5-8 times with deionized water to Neutral, constant temperature drying oven was dried at 110 C for 2 h, and the crude product was recrystallized from ethanol to give white solid 5-acetyl salicylamide. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping