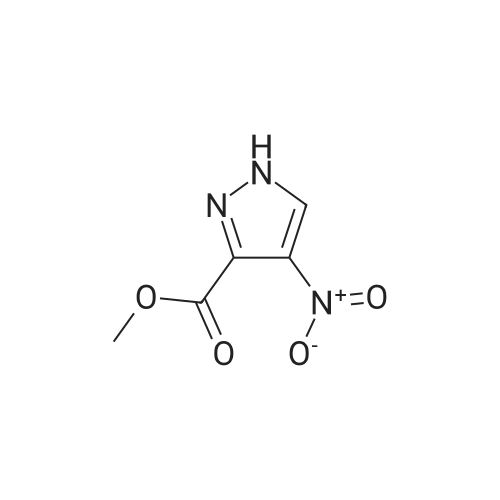
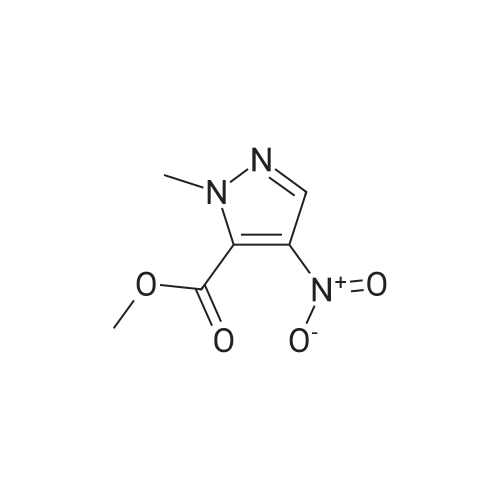
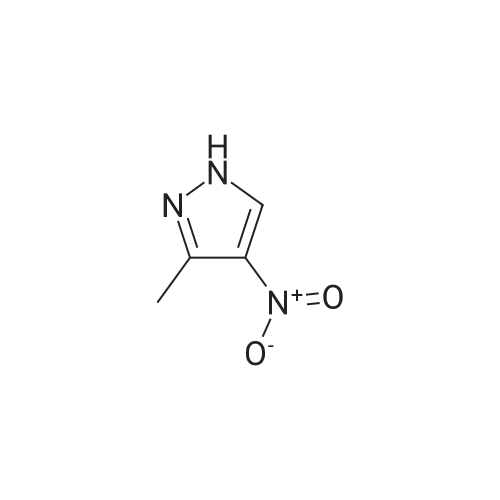
| 96% |
With hydrogen;palladium 10% on activated carbon; In methanol; under 3102.97 Torr; for 24h; |
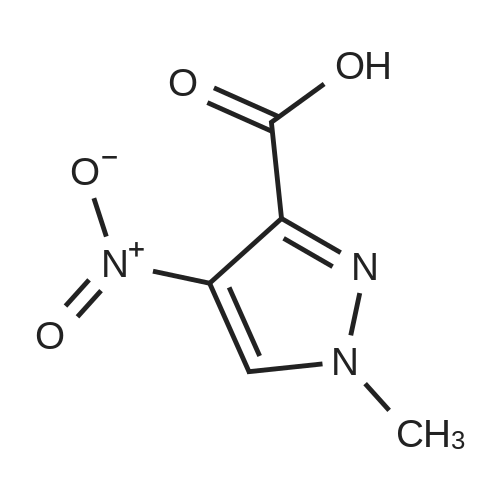
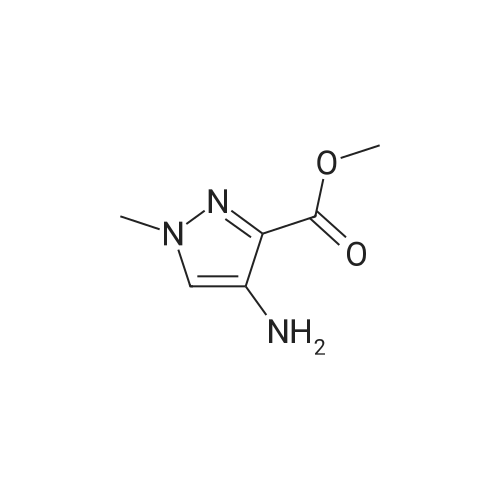
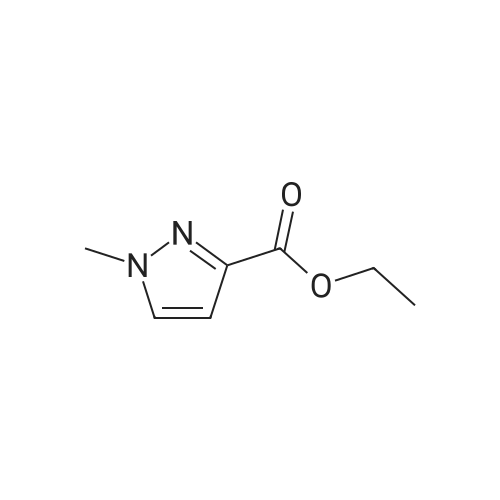
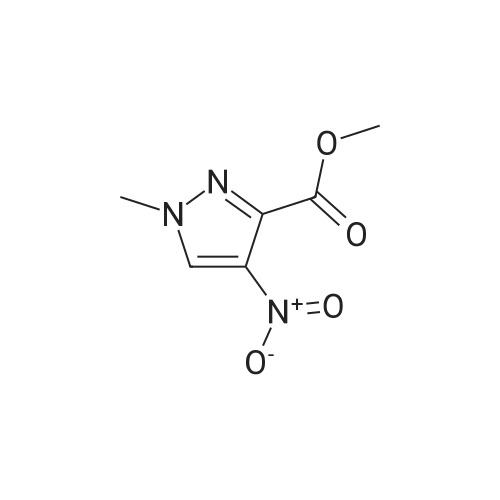
A mixture of [1-METHYL-4-NITRO-LH-PYRAZOLE-3-CARBOXYLIC] acid methyl ester (500 mg, 2.7 mmol) and 10% palladium on carbon [(50] mg) in methanol [(25] mL) was subjected to 60 psi pressure of hydrogen gas in a Parr apparatus for 24 h. At this time, the reaction mixture was filtered through a pad of celite and washed with methanol. The filtrate was concentrated in vacuo to afford [4-AMINO-1-METHYL-LH-PYRAZOLE-3-] carboxylic acid methyl ester (402 mg, 96%) as an off-white solid |
| 95% |
With hydrogen;palladium 10% on activated carbon; In methanol; under 30402.0 Torr; |
240 mg (1.30 mM) of the compound obtained in Preparative Example 7 was dissolved in 5 ml of methanol, to which 24 mg of 10% palladium/charcoal was added dropwise, and the resulting mixture was stirred under hydrogen pressure of 40 atm for 30 minutes. After the reaction was terminated, the resulting reaction solution was filtered through cellite, and distilled under reduced pressure, to obtain 191 mg (95%) of the title compound.1H NMR (300 MHz, CDCl3) delta 3.86 (s, 3H), 3.92 (s, 3H), 6.91 (s, 1H).Mass: 155 (M+) |
| 95% |
With palladium on activated charcoal; In methanol; at 25℃; for 1h; |
A solution of methyl 1-methyl-4-nitro-1H-pyrazole-3-carboxylate (1.0 g, 5.41 mmol) and Pd/C (200 mg) in MeOH (60 mL) was stirred for 1 hour at 25C. Pd/C was filtered out and the filtrate was concentrated to give desired compound as a white solid (800 mg, 95%). |
| 95% |
With hydrogen;palladium 10% on activated carbon; In methanol; under 30402.0 Torr; for 0.5h; |
240 mg (1.30 mM) of the compound obtained in Preparative Example 7 was dissolved in 5 ml of methanol, to which 24 mg of 10% palladium/ EPO <DP n="28"/>charcoal was added dropwise, and the resulting mixture was stirred under hydrogen pressure of 40 atm for 30 minutes. After the reaction was terminated, the resulting reaction solution was filtered through cellite, and distilled under reduced pressure, to obtain 191 mg (95%) of the title compound.1H NMR(SOOMHZ, CDCl3) delta 3.86(s, 3H), 3.92(s, 3H), 6.91(s, IH). Mass : 155(M+) |
| 84% |
With hydrogen;palladium 10% on activated carbon; In methanol; at 20℃;Inert atmosphere; |
A solution of 1-methyl-4-nitro-1H-pyrazole-3-carboxylic acid methyl ester (4.8 g, 25.94 mmol) in dry MeOH (100 ml) was thoroughly purged with argon, treated with 10% Pd-C (2.7 g, 2.59 mmol), and again purged with argon. Then the reaction mixture was hydrogenated under a balloon pressure of hydrogen at rt for overnight. The reaction mixture was filtered through a bed of celite. The filtrate was concentrated and dried to give the title compound as gray - whitesolid (3.4 g, 84%), which was used in the next reaction step without further purification. |
| 84% |
With hydrogen;palladium 10% on activated carbon; In methanol; at 20℃;Inert atmosphere; |
A solution of l-methyl-4-nitro-lH-pyrazole-3-carboxylic acid methyl ester (4.8 g, 25.94 mmol) in dry MeOH (100ml) was thoroughly purged with argon, treated with 10% Pd-C (2.7 g, 2.59 mmol), and again purged with argon. Then the reaction mixture was hydrogenated under a balloon pressure of hydrogen at rt for overnight. The reaction mixture was filtered through a bed of celite. The filtrate was concentrated and dried to give the title compound as gray-white solid (3.4 g, 84%), which was used in the next reaction step without further purification. |
|
With palladium 10% on activated carbon; hydrogen; In methanol; under 2068.65 Torr; for 1h; |
Methyl-4-nitro-lH-pyrazole-3-carboxylate (5.0 g, 27.01 mmol)Added to a hydrogenation reaction flask containing 50mL of methanol,Pd / C (10%, 0.5 g) was added,40psi hydrogen pressure reaction 1.0h,TLC test showed that the reaction was completed,Suction filtration, Pd / C filtration, and concentrated under reduced pressure to give the crude product (3.72 g, 88.7%),The crude product was used without purification in the next reaction administered. |
| 8.57 g |
With palladium 10% on activated carbon; hydrogen; In methanol; at 20℃; under 2327.23 Torr; for 6h; |
B) Methyl 4-amino-1-methyl-1H-pyrazole-3-carboxylate To a solution of methyl 1-methyl-4-nitro-1H-pyrazole-3-carboxylate (10 g) in methanol (200 mL), palladium-carbon (10%) (2 g) was added, and the mixture was stirred at room temperature for 6 hours in a hydrogen atmosphere (45 psi). Palladium-carbon was filtered off, and then, the filtrate was concentrated to obtain the title compound (8.57 g). 1H NMR (400 MHz, CDCl3) delta 3.86 (3H, s), 3.92 (3H, s), 4.08 (2H, brs), 6.96 (1H, s). |
|
With palladium 10% on activated carbon; hydrogen; In methanol; at 20℃; for 4h; |
10% palladium/C (2.87 g, 2.7 mmol) was added to a stirred solution of methyl 1-methyl- 4-nitro-1 H-pyrazole-3-carboxylate (p129, 7.15 g, 38.6 mmol) in methanol (250 ml_) and stirred at RT under H2 atmosphere for 4 hrs. The catalyst was filtered off and the solvent was evaporated under vacuum to afford methyl 4-amino-1-methyl-1 H-pyrazole- 3-carboxylate (p130, 6 g, y= quant) as purple wax used as such in the next step. MS (/T7/z): 156.1 [MH]+. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping