| 99% |
In dichloromethane; at 20℃; for 48h; |
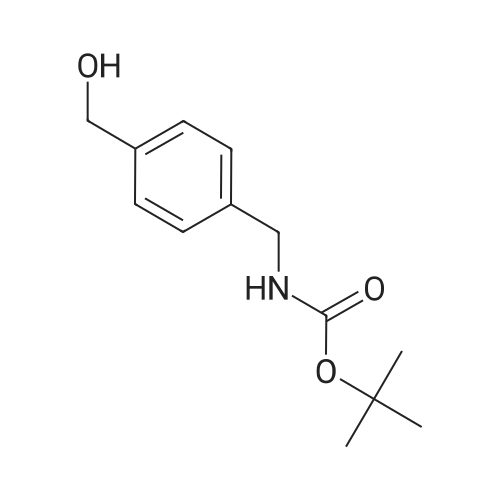
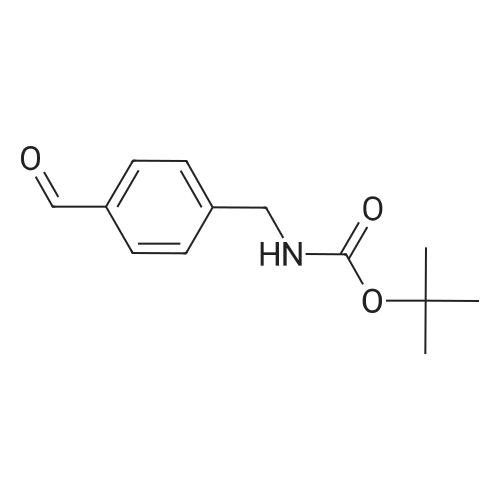
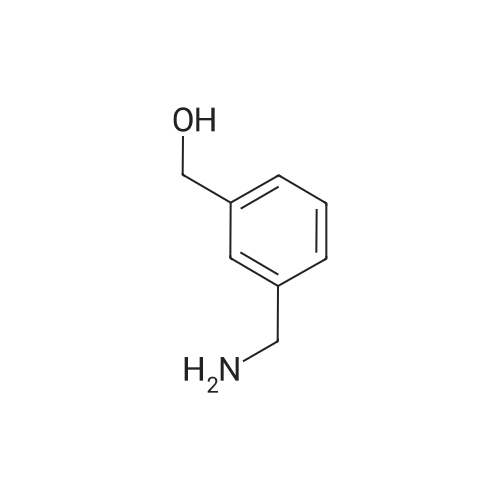
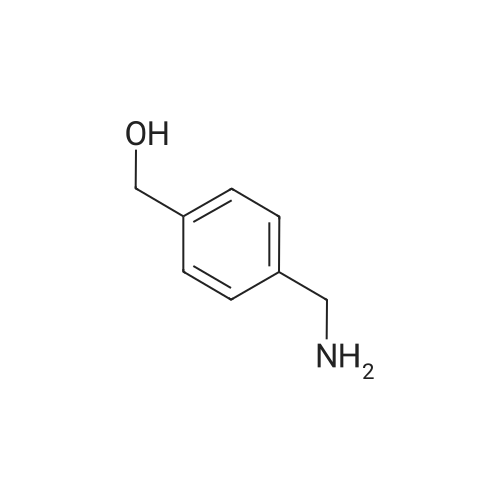
Boc2O was added in one portion at r.t. to a solution of (4-aminomethyl-phenyl)-methanol Compound 3a (21.2 mmol, 2.9 g) in CH2C12 (100 mL). The resulting solution wasstirred for 48h, then washed with a 10percent citric acid solution (50 mL) followed by brine. Theorganic layer was separated, then dried over Na2SC>4 and filtered. The solvent was removed invacua to obtain (4-hydroxymethyl-benzyl)-carbamic acid tert-butyl ester Compound 3b as awhite solid (5.2 g, 99percent yield), which was used in the next step without further purification.MnO2 (9.6 g) was added to a solution of Compound 3b (21.2 mmol, 5.2 g) inchloroform (60 mL), forming a black suspension that was stirred at r.t. overnight then filteredthrough a pad of celite. The solvent was evaporated in vacua to obtain (4-forinyl-benzyl)-carbamic acid tert-butyl ester Compound 3c as a white solid (4.3 g, 87percent yield), which was usedin the next step without purification.; NaB(OAc)3H (2.8 mmol, 0.58 g) was added to a mixture of Compound 3c (2.6 mmol,0.6 g) and tetrahydro-pyran-4-ylamine Compound 3d (2.6 mmol, 0.26 g) in CH2C12 (25 mL)and the resulting suspension was stirred at r.t. An aliquot of the reaction mixture showed theformation of product (MS m/e 321; 100percent). An aqueous solution of formaldehyde (37percentsolution, 8.6 mmol, 0.7 mL) was added to the reaction mixture, followed by NaB(OAc)3H (2.8mmol, 0.58 g) added in one portion under ice cooling. The reaction mixture was stirred at r.t.for about 2h, then made basic with a 2N NaOH solution and extracted with CH2C12. Theorganic layer was washed with brine, then separated and dried over Na2SO4. The drying agentwas filtered and the solvent was removed in vacua to yield (4-[methyl-(tetrahydro-pyran-4-yl)-amino]-methyl}-benzyl)-carbamic acid tert-butyl ester Compound 3e as a pale yellow oil.MS m/e 235 (M+H, 100percent). The product was purified by column chromatography (4:1CH2Cl2:MeOH) to yield a colorless oil (0.52 g, 59percent yield).; Compound 3e was dissolved in CH2Cl2, then HC1 in dioxane was added and themixture was stirred at r.t. for 12 hrs. The solvent was removed and the gummy residue wasmade basic with 2N NaOH and extracted with EtOAc. The organic layer was washed withbrine, then separated and dried over Na2SO4. The drying agent was filtered and the solvent wasremoved in vacua to obtain (4-aminomethyl-benzyl)-methyl-(tetrahydro-pyran-4-yl)-amineCompound 3f as a pale yellow oil (0.3 g, 83percent yield). MS m/e 235 (M+H, 100percent).; A solution of 3-(3-trifluoromethyl-phenyl)-acryloyl chloride Compound 3g (0.3 mrnol,0.07 g) in THF (2 mL) was added dropwise to a solution of Compound 3f (0.2 mmol, 0.05 g)and Et3N (0.8 mmol, 0.14 mL) in THF (10 mL) at 0°C. The resulting suspension was allowedto warm to r.t. overnight. The reaction mixture was made basic with a 2N NaOH solution andextracted with EtOAc (25 mL). The aqueous layer was extracted with EtOAc (2X10 mL) andthe organic layers were washed with brine, then dried over Na2SO4 and filtered. The solventwas removed in vacua to yield a yellow solid (with methane) as the product. The crude productwas purified by preparative TLC (9:1 EtOAc-.MeOH, Rf = 0.2) to yield N-(4-[methyl-(tetrahydro-pyran-4-yi)-amino]-methyl}-benzyi)-3-(3-trifluoromethyl-phenyl)-acrylamideCompound 3h (0.06 g, 49percent yield). MS m/e 433 (M+H, 100percent).; Mel (0.08 mL, 1.28 mmol) was added dropwise to a solution of Compound 3h (0.07mmol, 0.03 g) in a mixture of acetone:acetonitrile (2 mL). The resulting solution was stirred atr.t. for 24h to provide a residue. The residue was washed with ether (2x 1 mL) and dried undera high vacuum to provide Compound 64 (0.04 g, 93percent yield) as an iodide salt. MS m/e 584(M+H, 100percent). |
| 99% |
In dichloromethane; at 20℃; for 48h; |
Boc2O was added in one portion at r.t. to a solution of (4-aminomethyl-phenyl)-methanol Compound 3a (21.2 mmol, 2.9 g) in CH2Cl2 (100 mL). The resulting solution was stirred for 48 h, then washed with a 10percent citric acid solution (50 mL) followed by brine. The organic-layer was separated, then dried over Na2SO4 and filtered. The solvent was removed in vacuo to obtain (4-hydroxymethyl-benzyl)-carbamic acid tert-butyl ester Compound 3b as a white solid (5.2 g, 99percent yield), which was used in the next step without further purification. |
| 88% |
In chloroform;Inert atmosphere; |
1.7 l-'Butoxycarbonylaminomethyl-4-hydroxymethyl benzene, 7.7Amine 6 (1.44g, 10.5 mmol) was dissolved in CHCI3 (50 ml) and Boc20 (2.29 g, 10.5 mmol, 1 eq) was added slowly. The reaction was stirred under nitrogen overnight before the solvent was evaporated and the residue obtained was dissolved in ethyl acetate (50 ml). This solution was washed with a citric acid solution (3 * 50 ml), brine (50 ml), dried over Na2S04, and evaporated to yield 7 as a white solid (2.20 g, 9.2 mmol, 88 percent). Rf = 0.57 (DCM / MeOH sat. N3/4, 8:2); Vmax = cm-1; 'H NMR (300 MHz, CDC13) delta - 7.33 (d, 3J(H,H) = 8.2 Hz, 2H, ArCH a to CH2OH), 7.26 (d, J(H,H) = 8.2 Hz, 2H, ArCH a to CH2NHBoc), 4.86 (bs, 1H, NHBoc), 4.68 (s, 2Eta, CH^OH), 4.30 (d, 3J(H,H) = 5.7 Hz, 2H, CH?NHBoc), 1.96 (bs, 1H, OH), 1.46 (s, 9Eta, C(CH?)3); l 3C NMR (75 MHz, CDC13) delta = 155.9 (CO), 140.0 (ArCCH2OH), 138.3 (ArCCH2NHBoc), 127.6 (ArCH a to CH2NHBoc), 127.2 (ArCH a to CH2OH), 85.2 (C(CH3)3), 65.0 (C3/4OH), 44.4 (CH2NHBoc), 28.4 (C(C3/4)3); HRMS (ESI+): m/z calculated for C,3Hi9N03Na [M + Na]+ : 260.1257, found 260.1253. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping