| 95% |
With sulfuric acid; for 4h;Reflux; |
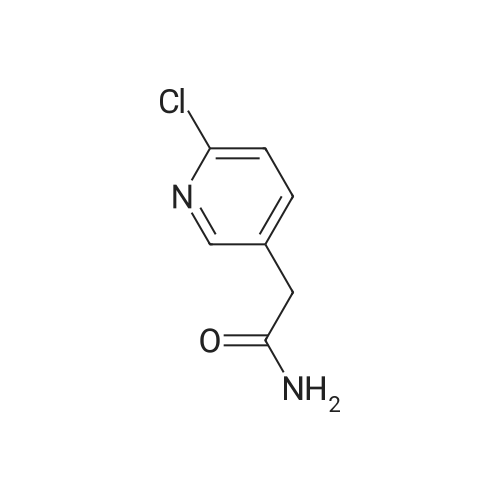
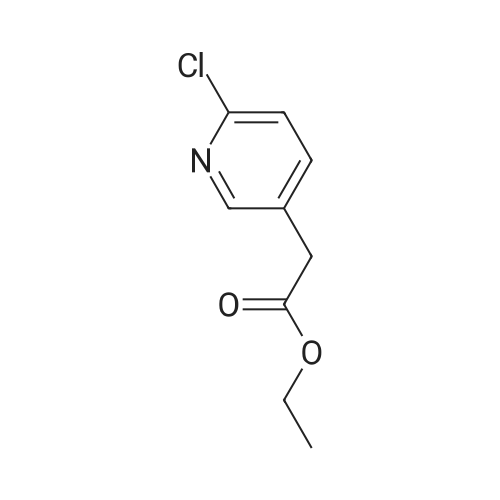
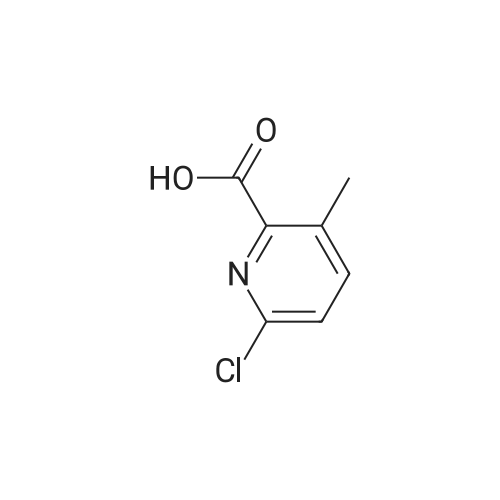
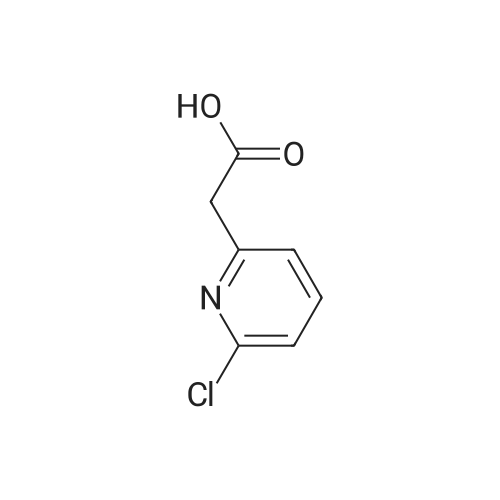
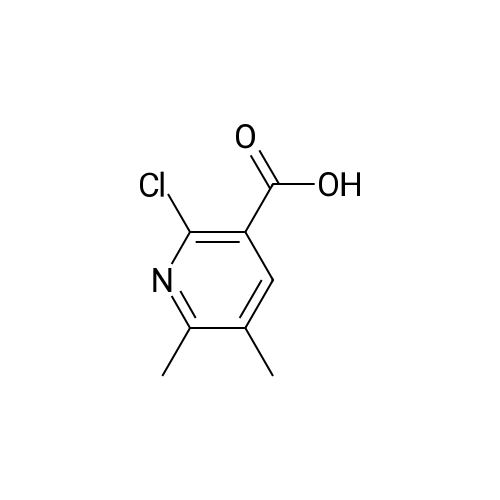
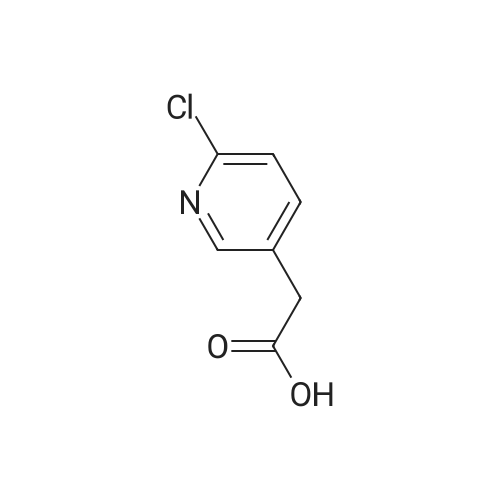
Step 1:[0815]To a solution of 6-chloro-3-pyridineacetic acid (1 g, 5.83 mmol) in ethanol was added sulfuric acid (1.6 mL). The mixture was refluxed for 4 h, then cooled to room temperature and concentrated. The residue was diluted with ethyl acetate and washed with a saturated sodium hydrogen carbonate solution. The resulting mixture was dried over magnesium sulfate and concentrated under reduced pressure to afford crude which was purified by column chromatography to afford ethyl 2-(6-chloropyridin-3-yl)acetate (1.1 g, 95%). |
| 87.2% |
With sulfuric acid; at 90℃; |
2-(6-chloropyridin-3-yl)acetic acid (4g, 22.4 mmol), ethanol (20 mL) and concentrated sulfuric acid (0.4 mL) were added to a 100 mL single-mouth bottle, and heated to 90 C to react overnight. After reaction, the mixture was cooled to room temperature, neutralized with saturated sodium bicarbonate, and extracted with ethyl acetate. The organic layers were combined, the solvent was dried with rotation under vacuum under an increased pressure, and the obtained product separated by a silica gel column (petroleum ether: ethyl acetate = 5:1) to give the product (colorless oil, 3.75 g), with a yield of 87.2%. 1H NMR (400 MHz, CDCl3) delta 8.28 (d, J= 1.5 Hz, 1H), 7.61 (dd, J= 8.2, 2.1 Hz, 1H), 7.29 (d, J = 8.2 Hz, 1H), 4.16 (q, J = 7.1 Hz, 2H), 3.59 (s, 2H), 1.25 (t, J = 7.1 Hz, 3H). |
| 79% |
|
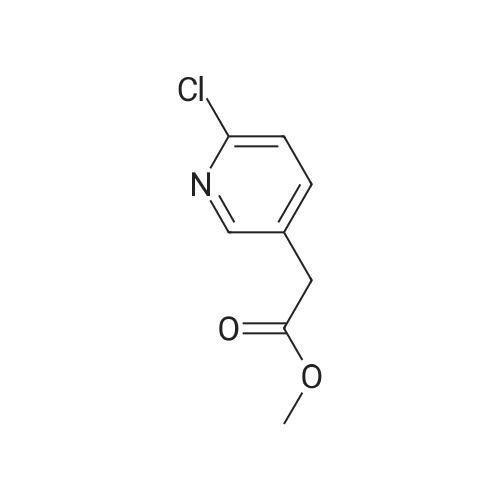
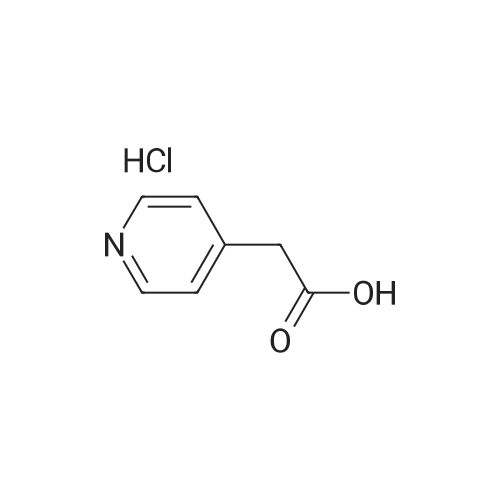
Example 28. Synthesis of K002. To a suspension of 6-cholro-2-pyridineacetic acid methyl ester (10 g, 58.2 mmol) was added cone, sulfuric acid (30 mL) and the reaction was heated to 70 C for 4 hr. The reaction mixture was cooled to room temperature and concentrated and the resulting residue was suspended in H20 (1L) and pH was adjusted to pH = 9 with sodium carbonate. The solution was extracted with ethyl acetate. The organics were washed with brine, dried over Na2S04, filtered and concentrated to give the crude product that was purified by flash column chromatography (30% EtOAC: 70% heptanes) to give the desired product 8.58 g, 79%.LC-MS: (ES, m/z): 186 [M+H]+ 1H-NMR: (400MHz, MeCN- J, ppm): delta 3.66 (m, 5H), 7.36(d, 1H), 7.67(d, 1H), 8.26(s, 1H). |
| 68.78% |
With sulfuric acid; for 4h;Reflux; |
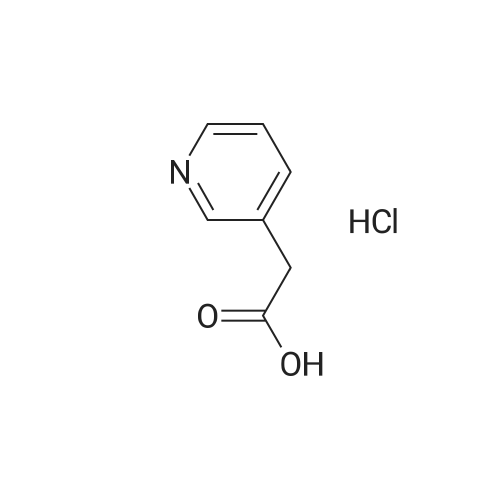
Synthesis of compound 74.2. To a solution of compound 74.1 (lOg, 0.0584mol, l .Oeq.) in ethanol (100 mL) was added drop wise H2SO4 (2mL). Reaction was stirred for 4 hours at reflux temperature. After completion of the reaction, solvent was evaporated and water was added. Mixture was extracted using ethyl acetate (150ml x2). Organic layer was washed with saturated sodium bicarbonate solution (70ml x 2), dried over sodium sulfate and concentrated under reduced pressure at 45C to afford compound 74.2 (8g, 68.78%). MS (ES): m/z 200.4 [M+H] + |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping