| 90% |
|
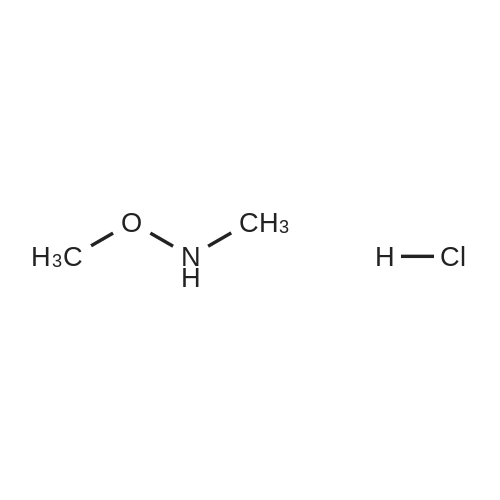
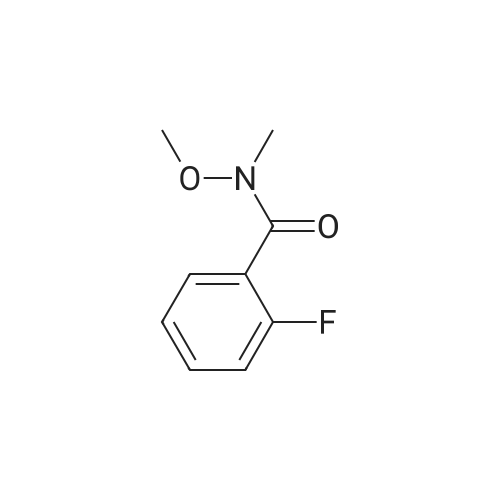
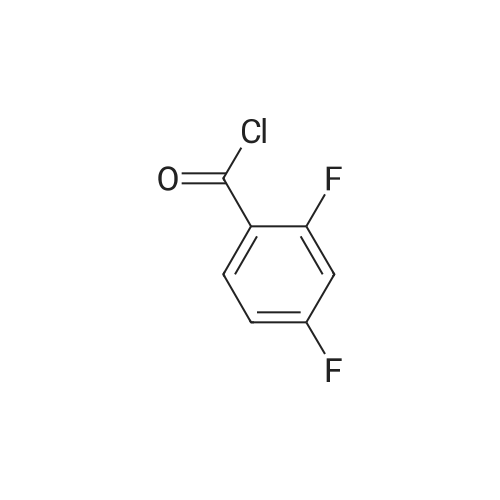
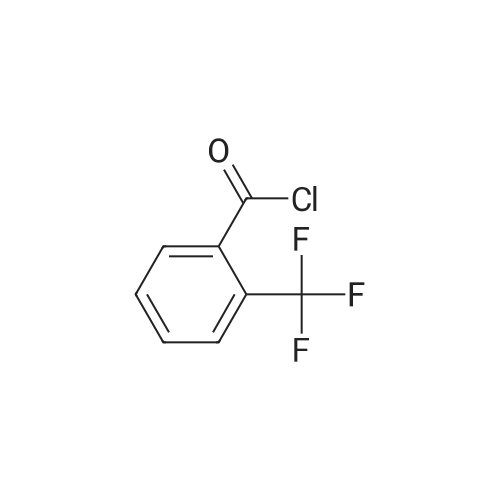
Triethylamine (5.32 mL, 37.84 mmol) was added dropwise to a solution of Nu,Omicron-dimethylhydroxylamine hydrochloride (2.768 g, 28.38 mmol) in anhydrous dichloromethane (45 mL) at 0 C. After stirring for 10 min, 2-fluorobenzoyl chloride (0.303 mL, 2.52 mmol) in anhydrous dichloromethane (15 mL) was added dropwise. The reaction mixture was returned to room temperature and stirred for 5 h. The reaction mixture was quenched with water (60 mL) and the products were extracted with dichloromethane (2 x 60 mL). The combined organic phases were washed with brine, dried over anhydrous sodium sulfate, and the solvent was removed under reduced pressure. Purification using flash chromatography (silica gel, hexanes:ethyl acetate, gradient 93:7 to 60:40) afforded 2-Fluoro-N-methoxy-N-methyl-benzamide (3.1 16, 17 mmol) in a 90% yield. [0 |
| 90% |
|
Triethylamine (5.32 mL, 37.8 mmol) was added dropwise to a solution of N,O-dimethylhydroxylamine hydrochloride (2.77 g, 28.4 mmol) in anhydrous dichloromethane (45 mL) at 0 C. After stirring for 10 min, 2-flurobenzoyl chloride (0.303 mL, 2.52 mmol) in anhydrous dichloromethane (15 mL) was added dropwise. The reaction mixture was returned to room temperature and stirred for 5 h. The reaction mixture was quenched with water (60 mL) and the products were extracted with dichloromethane (2 * 60 mL). The combined organic phases were washed with brine, dried over anhydrous sodium sulfate, and the solvent was removed under reduced pressure. Purification using flash chromatography (silica gel, hexanes/ethyl acetate, gradient 93:7 to 60:40) afforded 2-Fluoro-N-methoxy-N-methyl-benzamide (3.12 g, 17.0 mmol, 90% yield). 1H NMR (500 MHz, CDCl3): delta 7.44-7.38 (2H, m), 7.19 (1H, t, J = 7.5 Hz), 7.10 (1H, t, J = 8.9 Hz), 3.55 (3H, br s), 3.35 (3H, br s). 13C NMR (125 MHz, CDCl3): delta 166.40, 158.66, (d, J = 249 Hz), 131.50, 128.90, 124.11, 123.52 (d, J = 17 Hz), 115.69 (d, J = 21 Hz), 61.21, 32.31. 19F NMR (470 MHz, CDCl3): delta -114.04 (1F, s). HRMS (ESI) calculated for C9H10FNO2H+ (M+H)+ 184.07683, found 184.07702. |
| 62% |
With pyridine; In tetrahydrofuran; at 0 - 20℃; for 2.25h; |
N,O-Dimethylhydroxylamine hydrochloride (23.4 g, 240 mmol) was suspended inTHF (100 ml) and cooled to 0 C under nitrogen. Pyridine (32 ml, 400 mmol) was addedslowly. A solution of 2-fluorobenzoyl chloride (9.5 ml, 80 mmol) in THF (50 ml) was addedover 15 min. The reaction was removed from the ice bath and stirred at rt for 2 h. Water (100ml) and AcOEt (100 ml) were added, and the phases were separated. The aq. phase wasextracted with AcOEt (100 ml). The organic phases were pooled and washed with 1 N HCI (2x 100 ml) and 1 N NaOH (100 ml). After drying over MgSO4, the sample was concentrated toyield a yellow oil (10.7 g). The oil was purified by vacuum distillation, and a colorless oil wascollected (0.22 torr, 57-59 C, 9.1 g, 62% yield)2-fluoro-N-methoxy-N-methylbenzamide1H-NMR (300MHz, CDCI3) 53.34 (s, 3H), 3.54 (br, 3H), 7.10 (m, 1H), 7.19 (m, 1H),7.42 (m, 2H) |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science













 HazMat Fee +
HazMat Fee +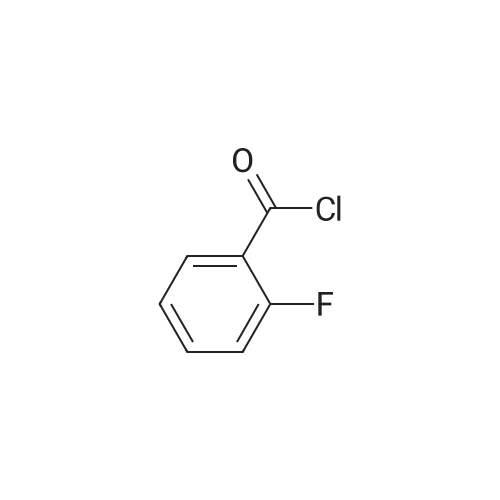

 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping