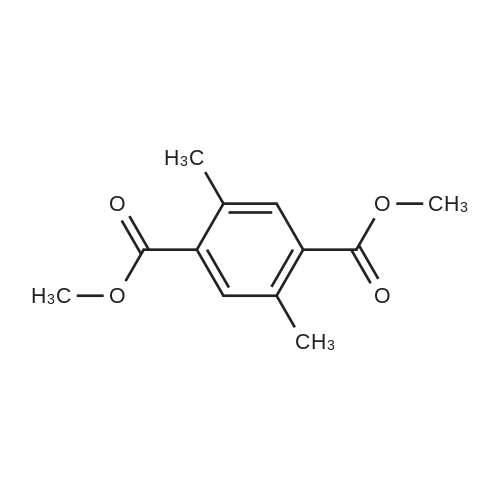
| 31.6% |
With N-Bromosuccinimide; 2,2'-azobis(isobutyronitrile); In tetrachloromethane; at 80℃; for 16h;Inert atmosphere; |
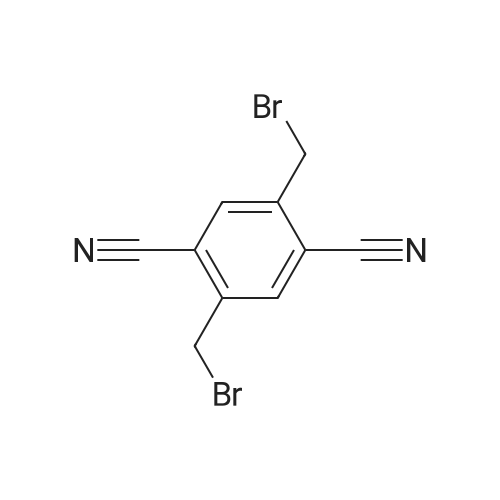
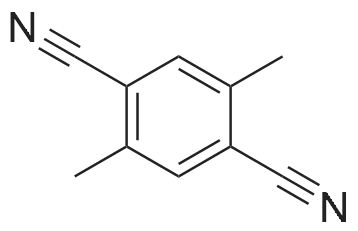
The <strong>[39095-25-5]2,5-dimethylterephthalonitrile</strong> (1.560 g, 10.0 mmol) was precisely weighed with an analytical balance.Azobisisobutyronitrile (0.082 g, 0.5 mmol) and NBS (3.916 g, 22.0 mmol) were placed in a dry 250 mL single port round bottom flask.Then use a measuring cylinder to measure 100 mL of carbon tetrachloride and pour it into the flask.Put on the reflux condenser,Vacuum and protect with argonPlace the flask in an oil bath heated to 80C or more and stir vigorously.And using a flame gun to assist in the rapid reflux,After rapid reflux, the temperature of the reaction system was adjusted to 80 C. for 16 hours. After the reaction is completed,The reaction mixture was cooled to room temperature and filtered.After washing with CCl 4 (3×20 mL), the filtrate was concentrated under reduced pressure to a brown-red viscous oil.Add ethanol (20 mL) to dissolve it slightly by heating.Transfer to a beaker (100 mL) and wash the flask with ethanol (3 x 10 mL).Pour the lotion into the beakerAfter leaving at room temperature to a total volume of 10 mL or less, it was filtered to obtain a white powdery product.The crude product was subjected to silica gel column chromatography [eluent: v (n-hexane): v (ethyl acetate) = 12:1].White powder, yield 31.6% (0.98g), |
|
With bromine; In water; |
2) Synthesis of 2,5-dibromomethyl-terephthalonitrile 2g of <strong>[39095-25-5]2,5-dimethyl-terephthalonitrile</strong> (0.0128mole), 1.9ml of bromine (0.384mole) and 100ml of dichoromethane were added to a middle pressure tube, and the reaction was performed at 60 for 24 hours. After completion of the reaction, 200ml of water was added thereto, and then the pH of the reaction solution was pH 10 with 2% sodium hydroxide. After the organic layer was separated, extracted by 200ml of water two times, and evaporated in vacuum. The concentrated crystal was loaded to column with hexane to obtain 1.1g of product (3.5mole, yield: 27.5%). |
| 0.57 g |
With N-Bromosuccinimide; 2,2'-azobis(isobutyronitrile); In tetrachloromethane; at 75℃; for 1h; |
In 100ml 4-neck reactor <strong>[39095-25-5]2,5-dimethyl-terephthalonitrile</strong> 2.3g, Carbon tetrachloride 23ml, NBS 5.3g, AIBN 0.07g 1 hour reaction at charged 75C It was. After completion of the reaction, it was allowed to cool to room temperature. Wash the residue and the reaction solution was filtered with carbon tetrachloride It was. Crude product is obtained in the oil and the filtrate was concentrated. The addition of methanol and allowed to crystallize the crude product. Taken out crystals were filtered off, 1,4-dicyano-2,5-bis-bromo methyl benzene was obtained 0.57g. In 50ml 3-necked reactor 1,4-dicyano-2,5-bis bromomethylbenzene 0.55g, Triethyl phosphite 0.61g was stirred and heated to charged 100-110 . At this temperature the reaction was carried out for 2 hours. Was allowed to cool to room temperature, and purified on a silica gel column 2,5-dicyano-1,4-bis (diethyl phosphonyl methyl) benzene was obtained 0.45g. 50ml 3-necked reactor to 2,5-dicyano-1,4-bis (diethyl phosphonyl methyl) benzene 0.45g, DMF (dehydration) 15ml, 4 formylpyridine 0.28g They were charged. After nitrogen substitution, Potassium t- butoxide 0.35g It was changed to green-brown solution and dropping the 5mlDMF solution slowly. It was allowed to react for 3 hours at 60 . The reaction mixture was extracted with chloroform and released into the water. The reaction mixture was concentrated to give crude product. The crude product was purified on a silica gel column (toluene / acetone 1/1) and the resulting solid to yield 0.08g of intermediate 45 and purified construed methanol. A synthesis flow of this intermediate 45 below |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping