| 70% |
With sodium hydroxide;tetrakis(triphenylphosphine) palladium(0); In tetrahydrofuran; at 70℃;Inert atmosphere; |
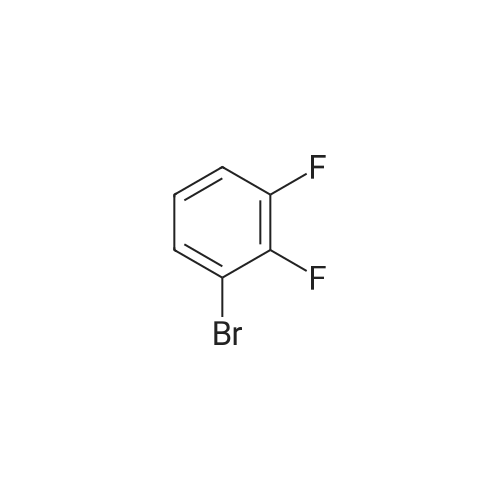
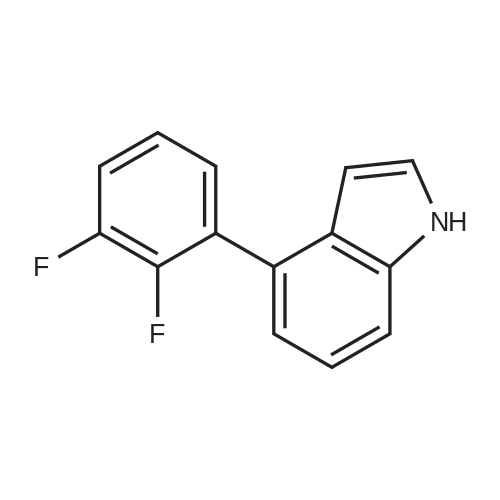
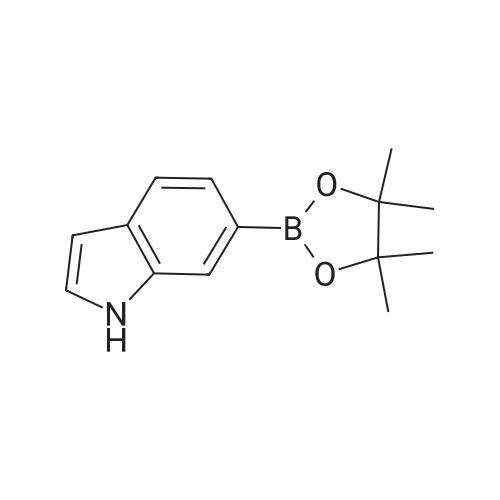
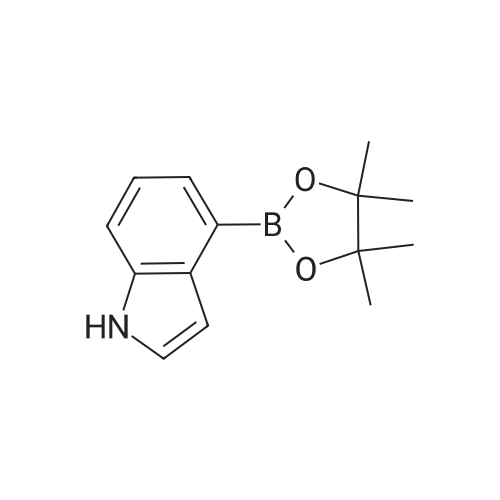
Step 2 4-(2,3-Difluoro-phenyl)-1H-indole 4-(4,4,5,5-Tetramethyl-[1,3,2]dioxaborolan-2-yl)-1H-indole 6b (1.22 g, 5 mmol) was dissolved in 20 ml of tetrahydrofuran under stirring, and added with <strong>[38573-88-5]1-bromo-2,3-difluoro-benzene</strong> (0.97 g, 5 mmol), tetrakis (triphenylphosphine)palladium (0.17 g, 0.15 mmol) and sodium hydroxide solution (7 ml, 2 mol/L) under an argon atmosphere. Upon completion of the addition, the reaction system was stirred at 75° C. in an oil bath overnight. After thin lay chromatography showed the disappearance of starting materials, the reaction mixture was naturally cooled down to room temperature and extracted with ethyl acetate (20 ml*3). The combined organic extracts were washed with saturated brine (10 ml), dried over anhydrous sodium sulfate, filtered to remove the drying agent and concentrated under reduced pressure. The residue was purified by silica gel column chromatography to obtain 4-(2,3-difluoro-phenyl)-1H-indole 6c (800 mg, yield 70percent) as a white solid. MS m/z (ESI): 228.4 [M-1] |
| 70% |
With sodium hydroxide;tetrakis(triphenylphosphine) palladium(0); In tetrahydrofuran; at 75℃;Inert atmosphere; |
4-(4,4,5,5-Tetramethyl-[1,3,2]dioxaborolan-2-yl)-1H-indole 6b (1.22 g, 5 mmol) was dissolved in 20 ml of tetrahydrofuran under stirring, and added with <strong>[38573-88-5]1-bromo-2,3-difluoro-benzene</strong> (0.97 g, 5 mmol), tetrakis (triphenylphosphine)palladium (0.17 g, 0.15 mmol) and sodium hydroxide solution (7 ml, 2 mol/L) under an argon atmosphere. Upon completion of the addition, the reaction system was stirred at 75°C in an oil bath overnight. After thin lay chromatography showed the disappearance of starting materials, the reaction mixture was naturally cooled down to room temperature and extracted with ethyl acetate (20 ml.x.3). The combined organic extracts were washed with saturated brine (10 ml), dried over anhydrous sodium sulfate, filtered to remove the drying agent and concentrated under reduced pressure. The residue was purified by silica gel column chromatography to obtain 4-(2,3-difluoro-phenyl)-1H-indole 6c (800 mg, yield 70percent) as a white solid. MS m/z (ESI): 228.4[M-1] |
| 70% |
With sodium hydroxide;tetrakis(triphenylphosphine) palladium(0); In tetrahydrofuran; at 75℃;Inert atmosphere; |
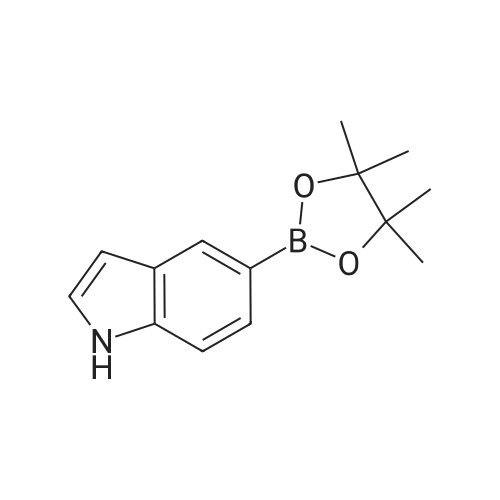
4-(4,4,5,5-Tetramethyl-[1,3,2]dioxaborolan-2-yl)-1H-indole 3b (1.22 g, 5 mmol) was dissolved in 20 mL of tetrahydrofuran under stirring under an argon atmosphere, and <strong>[38573-88-5]1-bromo-2,3-difluoro-benzene</strong> (0.97 g, 5 mmol), tetrakis (triphenylphosphine) palladium (0.17 g, 0.15 mmol) and 7 mL of sodium hydroxide solution (2M) were then added to the solution. Upon completion of the addition, the reaction system was stirred at 75°C in an oil bath overnight. The reaction was completed until TLC showed the disappearance of starting materials. The reaction mixture was naturally cooled down to room temperature and extracted with ethyl acetate (20 mL*3). The combined organic extracts were washed with saturated brine (10 mL), dried over anhydrous sodium sulfate, filtered to remove the drying agent and concentrated under reduced pressure. The resulting solid was purified by silica gel column chromatography to give the title compound 4-(2,3-difluoro-phenyl)-1H-indole 3c (800 mg, yield 70percent) as a white solid. MS m/z (ESI): 228.4[M-1] |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping