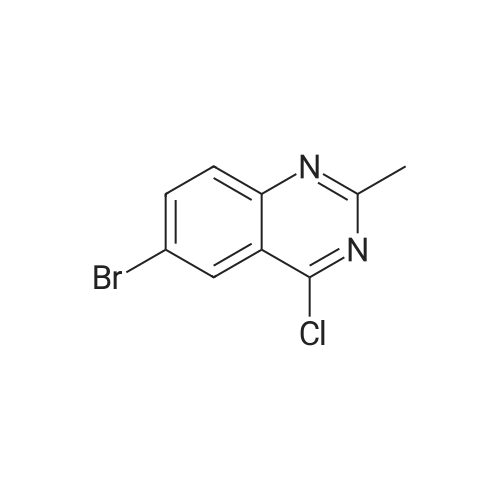
| 43% |
In isopropyl alcohol; at 80℃;Inert atmosphere; |
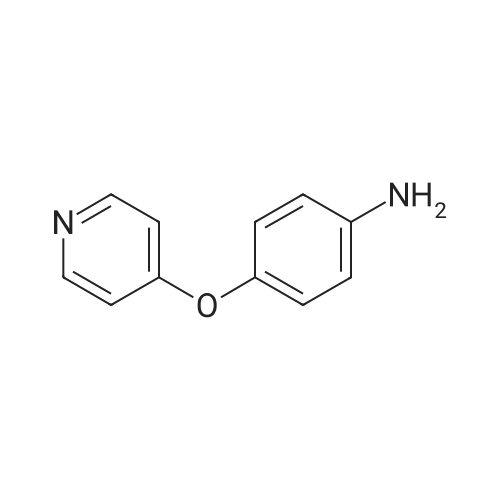
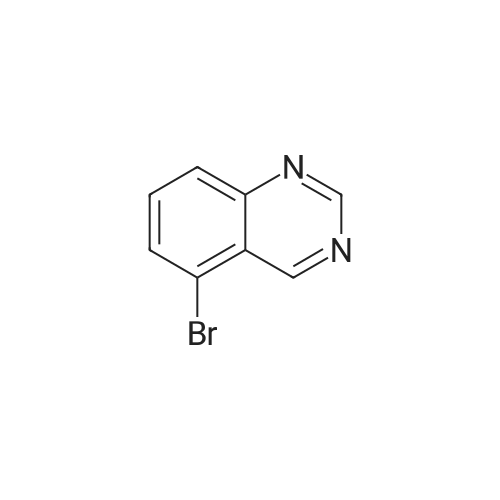
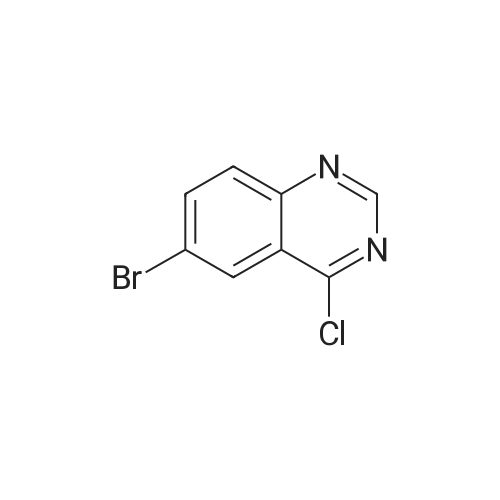
To a solution consisting of 6-bromo-4-chloroquinazoline (0.448 g, 1.84 mmol) in 2- propanol (10 mL) was added <strong>[102877-78-1]4-(pyridine-4-yloxy)aniline</strong> (0.360 g, 1.93 mmol). The reaction mixture was heated (80 C) and stirred overnight under a flow of N2. The reaction mixture was cooled to room temperature and then the reaction mixture was filtered over a fritted funnel. The filtered solid was rinsed with excess 2-propanol and dried under high vacuum to afford 6-bromo-N-(4-(pyridin-4-yloxy)phenyl)quinazolin-4-amine (3F) as an off-white solid (313 mg, 43% yield,97% purity). MS (ESI + m/z 394.0, ESI - m/z 392.0). Next a solution consisting of 6-bromo-N- (4-(pyridin-4-yloxy)phenyl)quinazolin-4-amine (0.306 g, 0.77 mmol) in anhydrous ethanol (10 mL) was placed in a 20 mL microwave reaction vial containing a stir bar. Next, 3- aminopyridine-5- boronic acid pinacol ester (6, 0.176 g, 0.80 mmol) was added followed bySiliCatDPP-Pd (5 mol %, 0.26mmol/g loading, 0.150 g) and 10% aqueous potassium carbonate solution (2 equivalents, 1.15 mL, 1.6 mmol). The reaction mixture was placed under N2 atmosphere, capped, and then heated at 125 C for one horn in a Biotage Emrys Optimizer microwave. The reaction mixture was allowed to cool to room temperature and then filtered over a fritted funnel to collect SiliCat DPP-Pd. The filtered solid was rinsed with excess ethanoland the filtrate was concentrated under reduced pressure to afford the crude product.Purification of the crude product by Biotage Isolera flash chromatography using a gradient of 4-100% ethyl acetate in heptane, followed by 0-10% methanol in dichloromethane afforded 7F 6-(5-aminopyridin-3-yl)-N-(4-(pyridin-4-yloxy)phenyl)quinazolin-4-amine (50 mg, 15% yield,92% purity) as an off-white solid. MS (ESI + m/z 407.1, ESI m/z 405.1). To a roomtemperature solution of 6-(5 -aminopyridin-3 -yl)-N-(4-(pyridin-4-yloxy)phenyl)quinazolin-4- amine (50 mg, 0.12 mmol) in pyridine (3 mL) was added methanesulfonyl chloride (56 mg, 0.5 mmol). The reaction mixture turned dark red which persisted and was stirred for 15 minutes. The reaction mixture was poured into a saturated solution of sodium bicarbonate and the organic material was extracted with ethyl acetate. The organic phase was washed with water and brine, dried over magnesium sulfate, filtered and concentrated under vacuum. The cmde solid was dissolved in methanol and ?dry loaded? on to a silica column eluted with a gradient of 1/9 to 3/7methanol/ethyl acetate to give N-(5-(4-((4-(pyridin-4-yloxy)phenyl)amino)quinazolin-6- yl)pyridin-3-yl)methanesulfonamide (5F, MOL-166, 20 mg, 33% yield, 96% purity) as a solid. ?H NMR (400MHz, DMSO-d6) oe 10.07 (s, 1H), 8.91 (s, 1H), 8.79 (d, J=1.9 Hz, 1H), 8.62 (s, 1H), 8.4-8.5 (m, 3H), 8.15 (dd,J=1.7, 8.6 Hz, 1H), 7.85-8.0(m, 4H), 7.24 (d,J8.9Hz, 2H),6.94 (d, J=4.7 Hz, 2H), 3.08 (s, 3H); MS: (ESI + m/z 485.1, ESI- m/z 483.0). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping