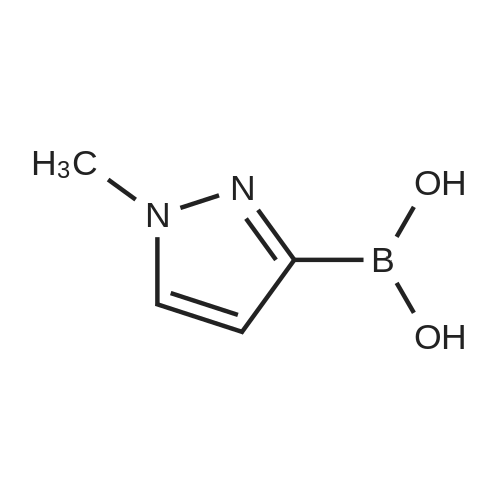
| 77% |
With triethylamine;(1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride; In 1,2-dimethoxyethane; water; at 20℃; for 4h;Heating / reflux; |
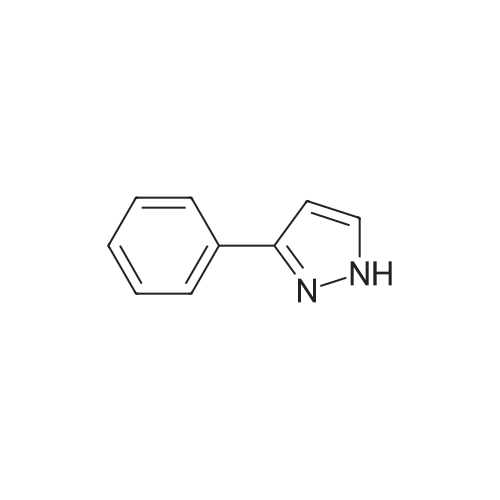
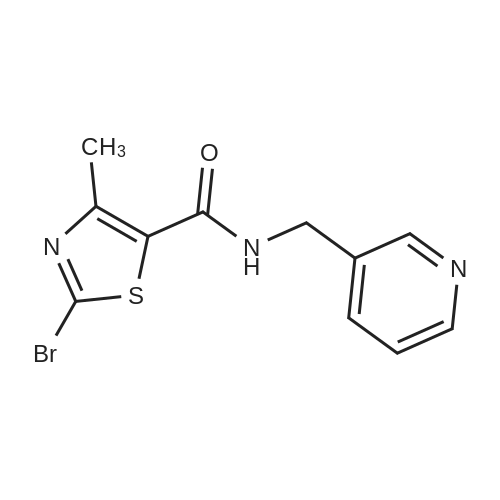
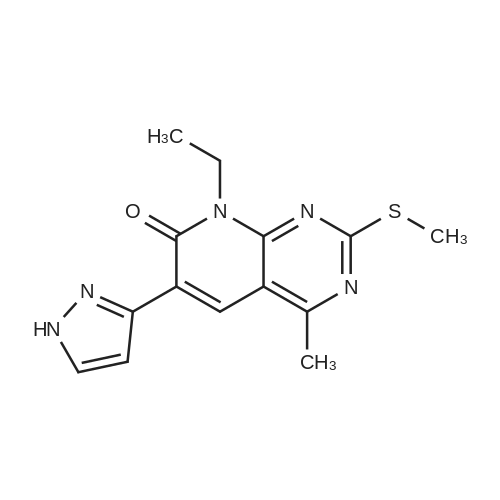
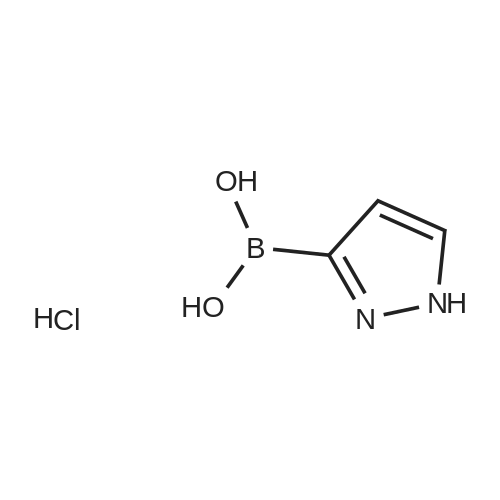
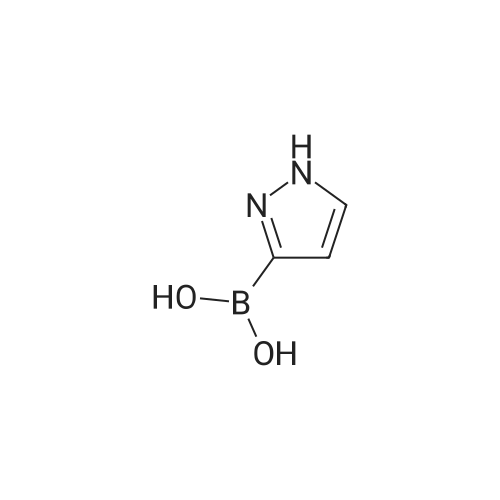
[00207] To a solution of 6-bromo-8-ethyl-4-methyl-2-(methylthio)pyrido[2,3-d]pyrimidin- 7(8H)-one (0.765 g, 2.43 mmol) in DME-H2O (10:1 11 mL) was added lH-pyrazol-5- ylboronic acid (Frontier, 0.408 g, 3.65 mmol), [1,1'- bis(diphenylphosphino)ferrocene]dichloropalladium(II) complex with CH2Cl2 (Pd(dpprhof),0.198 g, 0.243 mmol) and triethylamine (0.736 g, 7.29 mmol) at room temperature. Then the reaction mixture was heated to reflux and reacted for 4 h. After cooling down to room temperature, the reaction mixture was partitioned with water and ethyl acetate. After separation, the organic layer was dried with Na2SO4, and the product 8-ethyl-4-methyl- 2-(methylthio)-6-(lH-pyrazol-5-yl)pyrido[2,3-d]pyrimidin-7(8H)-one (0.567 g, 77percent yield) was obtained by silica gel column chromatography. 1H NMR (400 MHz, CDCl3): delta 13.3 (bs, IH), 8.54 (s, IH), 7.82-7.07 (m, 2H), 4.45 (q, J= 7.2 Hz, 2H), 2.71 (s, 3H), 2.60 (s, 3H), 1.26 (t, J= 7.2Hz, 3H). EPO <DP n="66"/> |
| 77% |
With triethylamine;dichloro(1,1'-bis(diphenylphosphanyl)ferrocene)palladium(II)*CH2Cl2; In 1,2-dimethoxyethane; water; at 20℃; for 4h;Heating / reflux; |
To a solution of 6-bromo-8-ethyl-4-methyl-2-(methylthio)pyrido[2,3-d]pyrimidin- 7(8H)-one (0.765 g, 2.43 mmol) in DME-H2O (10: 1 1 1 mL) was added l H-pyrazol-5-ylboronic acid (Frontier, 0.408 g, 3.65 mmol), [1,1 '- bis(diphenylphosphino)ferrocene]dichloropalladium(II) complex with CH2Cl2 (Pd(dpppf),0.198 g, 0.243 mmol) and triethylamine (0.736 g, 7.29 mmol) at room temperature. Then the reaction mixture was heated to reflux and reacted for 4 h. After cooling down to room temperature, the reaction mixture was partitioned with water and ethyl acetate. After separation, the organic layer was dried with Na2SO4, and the product 8-ethyl-4-methyl-2-(methylthio)-6-(lH-pyrazol- 5-yl)pyrido[2,3-d]pyrimidin-7(8H)-one (0.567 g, 77percent yield) was obtained by silica gel column chromatography. 1H NMR (400 MHz, CDCl3): delta 13.3 (bs, IH), 8.54 (s, IH), 7.82-7.07 (m, 2H), 4.45 (q, J = 7.2 Hz, 2H), 2.71 (s, 3H), 2.60 (s, 3H), 1.26 (t, J = 7.2Hz, 3H). |
| 77% |
With triethylamine;dichloro(1,1'-bis(diphenylphosphanyl)ferrocene)palladium(II)*CH2Cl2; In 1,2-dimethoxyethane; water; at 20℃; for 4h;Heating / reflux; |
[00242] To a solution of 6-bromo-8-ethyl-4-methyl-2-(methylthio)pyrido[2,3-d]pyrimidin- 7(8H)-one (0.765 g, 2.43 mmol) in DME-H2O (10:1 11 mL) was added lH-pyrazol-5- ylboronic acid (Frontier, 0.408 g, 3.65 mmol), [1,1 '- bis(diphenylphosphino)ferrocene]dichloropalladium(II) complex with CH2Cl2 (Pd(dpppf),0.198 g, 0.243 mmol) and triethylamine (0.736 g, 7.29 mmol) at room temperature. Then the reaction mixture was heated to reflux and reacted for 4 h. After cooling down to room temperature, the reaction mixture was partitioned with water and ethyl acetate. After separation, the organic layer was dried with Na2SO4, and the product 8-ethyl-4-methyl- <n="74"/>2-(methylthio)-6-(lH-pyrazol-5-yl)pyrido[2,3-d]pyrimidin-7(8H)-one (0.567 g, 77percent yield) was obtained by silica gel column chromatography. 1H NMR (400 MHz, CDCl3): delta 13.3 (bs, IH), 8.54 (s, IH), 7.82-7.07 (m, 2H), 4.45 (q, J= 7.2 Hz, 2H), 2.71 (s, 3H), 2.60 (s, 3H), 1.26 (t, J= 7.2Hz, 3H). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping