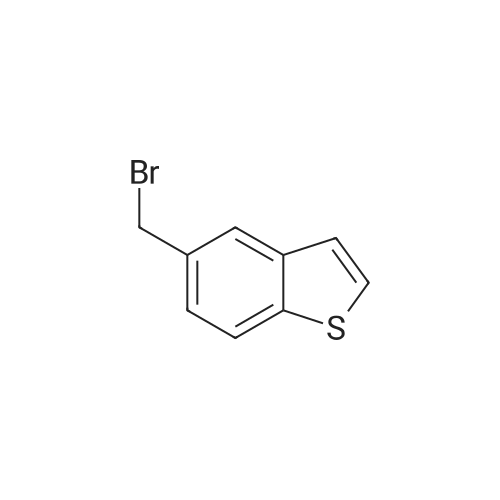
| 98.0% |
With acetic acid; at 10 - 80℃; for 6.5h; |
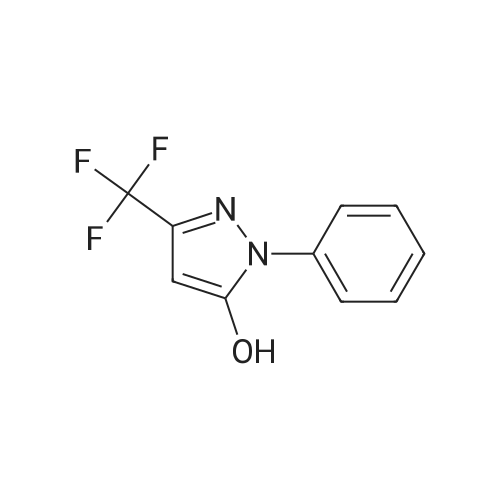
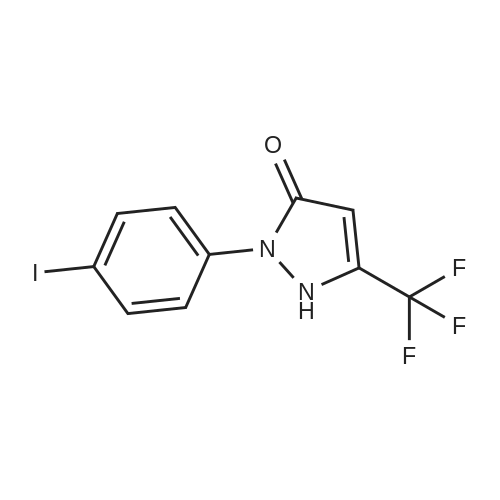
(Reference Example 2) Synthesis of 5-hydroxy-1-phenyl-3-trifluoromethylpyrazole: Ethyl 4,4,4-trifluoroacetoacetate (18.4 g (0.1 mol)) was dissolved in 12.0 g (0.2 mol) of acetic acid. The resulting solution was cooled to 10C or less with stirring, and 11.8 g (0.11 mol) of phenylhydrazine was added dropwise thereto over 0.5 hours. After the dropwise addition, the solution was stirred at room temperature for 1 hour and subsequently at 80C for 5 hours. When the reaction was completed, the reaction solution was cooled to room temperature, and 100 mL of water was added thereto. The produced crystal was collected by filtration, washed twice with 50 mL of water and dried by a hot air drier to obtain 22.3 g (yield: 98.0%) of the title compound as a pale yellow crystal. LC-MS(EI): m/z=228 (M+), melting point: 190-192C. |
| 92% |
In ethanol; for 12h;Reflux; |
Ethyl 4, 4, 4-1rifluoro3-oxobutanoate (S3, 200 p1., 1.37 mmoi, I equiv) and phenyihydrazine (148 IrL. 1.37 inmol, 1.00 equiv) were dissolved in ethanol (1.4 mL) and the resulting mixture was stirred for 12 hours at reflux. The reaction mixture was cooled to 24 c and the solvent was evaporated in vacuo. The residue was dissolved into ethyl acetate (3 mL) and washed with IN HCI (3 x 3 mL). The organic layer was dried over sodium sulfate, filtrated and concentrated in vacuo. The resulting material was washed with dichloromn ethane (5 mL) to afford the compound as orange solid (289 mg, 92%).Rf 0.25 (30% ethyl acetate-hexanes; UV). ?HNMR (500 MHz, DMSOd6): d 7.71 (d, 2H, H2, J = 8.0 Hz), 7.51 (dd, 2H, J = 8.0 Hz, H3), 7.38 (t, IH, J = 8.0 Hz, H4), 5.94 (s, 1Ff, H,). ?3C NMR (125 MHz, DMSOd6): oe 153.7 (C), 140.4 (q, 2JCF 37.4 Hz, C), 137.7 (C), 129.1 (CH), 127.2 (CH), 122.3 (CH), 121.3 (q, IJCF 266.9Hz, CF3), 85.6 (q, 3JCF =1.6 Hz, CH). ?9F NMR (375 MHz, DMS&-do): 61.8. IR (ATR.FTIR), cm?1: 3373 (br),1599 (in), 1505 (in), 1491 (m), 1456 (m), 1407(m), 1151 (s), 1119 (s), 984 (s), 758 (s), 691(s). HRMSESI (m/z): [M+H1 calculated for C10H8F3N50, 229.0583; found, 229.0598. |
| 87.9% |
With hydrogenchloride; In ethanol; water; for 1h;Heating / reflux; |
20 g (184.9 mmoles) of phenylhydrazine and 4 ml of concentrated hydrochloric acid were added to a solution of 34.1 g (184.9 mmoles) of ethyl trifluoroacetoacetate dissolved in 500 ml of ethanol. The mixture was refluxed for 1 hour with heating, to give rise to a reaction. After the completion of the reaction, the reaction mixture was subjected to vacuum distillation to remove the most part of the solvent contained therein. The residue was mixed with water to precipitate crystals. The crystals were collected by filtration, washed with water until the filtrate became neutral, and dried to obtain 37.1 g (yield: 87.9%) of 1-phenyl-3-trifluoromethyl-1H-pyrazol-5-ol as ocherous crystals. 1H-NMR [CDCl3/TMS, delta (ppm) ]: 7.68-7.41 (5H,m), 5.86 (1H,s), 3.71 (1H,s) |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science













 HazMat Fee +
HazMat Fee +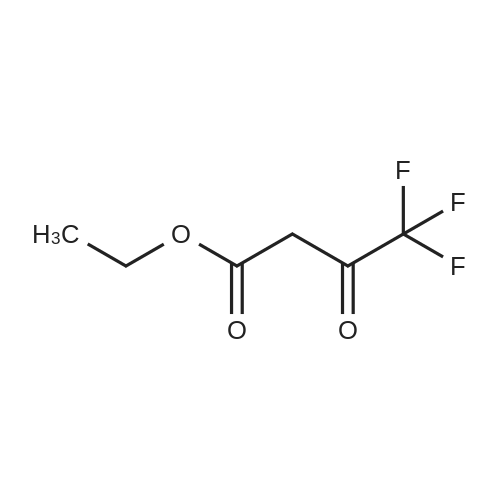

 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping