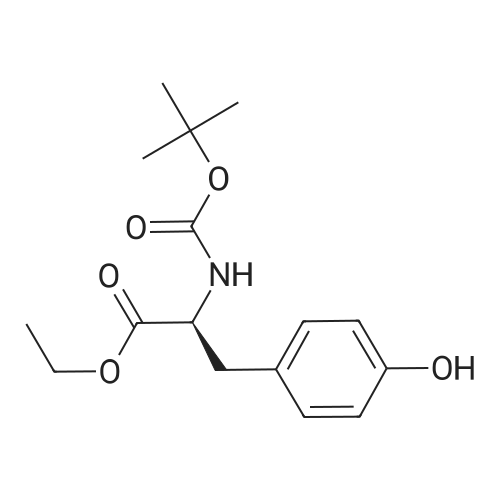
| 96% |
With sodium hydrogencarbonate; In tetrahydrofuran; water; at 0 - 20℃; for 16h; |
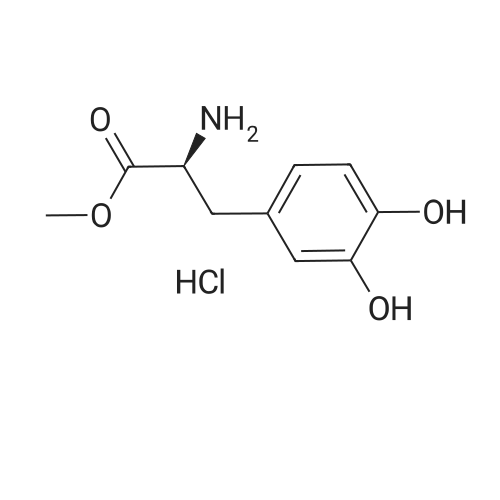
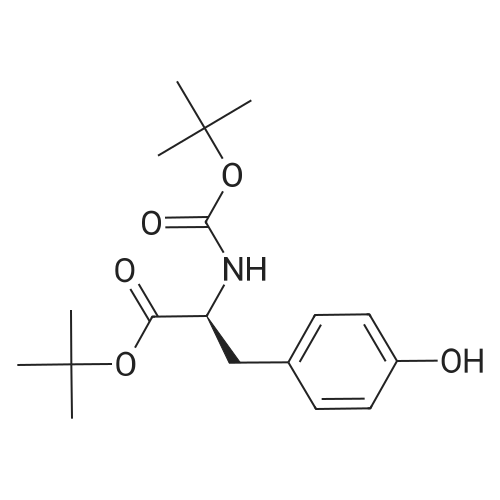
At 0 C,Sodium bicarbonate (50.4 g, 0.6 mol) was slowly added to (S)-2-amino-3-(3,4-methylenedioxyphenyl)propanoic acid methyl ester hydrochloride (74.3 g, 0.3 mol) In a 300 mL aqueous solution, a solution of Boc2O (74.3 g, 0.3 mol) in tetrahydrofuran (150 mL) was slowly added dropwise to the mixture. After the addition was completed, the mixture was gradually warmed to room temperature and stirred for 16 hours. Most of the solvent was removed by concentration, and then extracted with ethyl acetate. Water and brine were washed and dried over anhydrous sodium sulfate.Concentration gave the product (S)-methyl 2-(tert-butoxycarbonylamino)-3-(3,4-methylenedioxyphenyl)propanoate (ie compound 8) (89.6 g) The yield was 96%. |
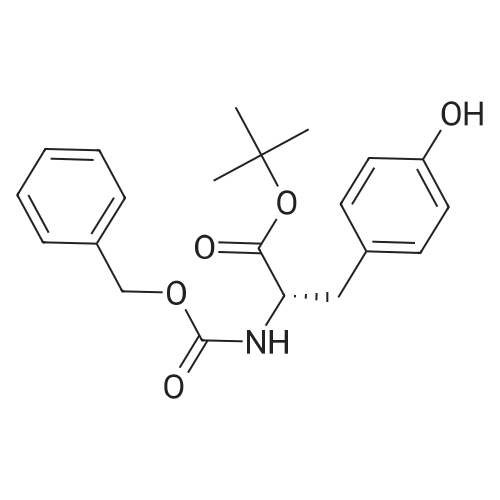
| 85% |
With sodium hydrogencarbonate; In tetrahydrofuran; water; at 20℃; for 1h; |
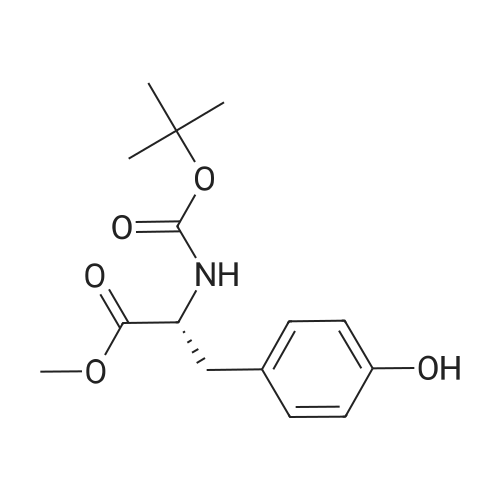
Toan ice-cold solution of 6 (0.9 g,3.6 mmol) in THF (6.5 mL), a saturated aqueous solution of NaHCO3(6.5 mL) and a solution of di-tert-butyl-dicarbonate (0.87 g, 4.0 mmol) in THF (4.0 mL) were addedsuccessively. The resulting reaction mixture was stirredat ambient temperature for 1 h and then evaporated to remove organic solvent.The aqueous phase was extracted thrice with CH2Cl2. Thecombined organic layers were washed with brine, dried over Na2SO4and evaporated to dryness. The residue was triturated with Et2O andrefrigerated. Compound 7was obtainedafter filtration under vacuo. |
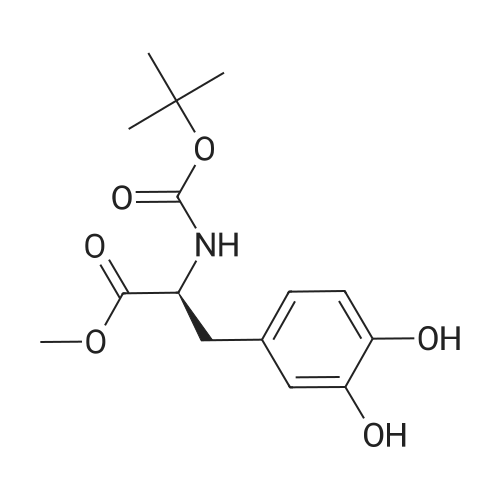
| 76% |
With sodium hydrogencarbonate; In tetrahydrofuran; water; for 1.5h;Cooling with ice; |
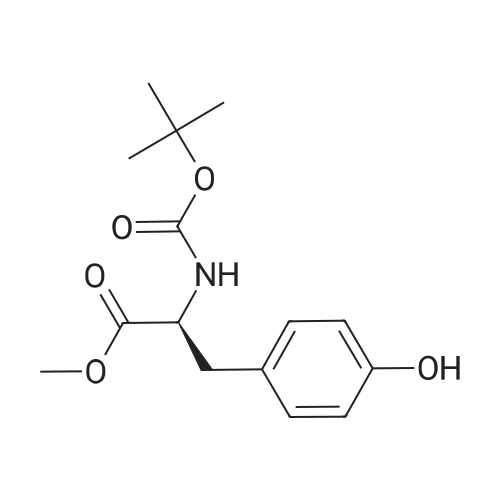
DOPA-OMe hydrochloride (1.26 g, 5.11 mmol) was dissolved in tetrahydrofuran (10 ml), saturated aqueous sodium bicarbonate solution (8 ml) was added, and the mixture was cooled in an ice bath. To this solution was added BocO (1.00 ml, 4.35 mmol), and the mixture was warmed to room temperature and stirred for 1.5 hr. The reaction mixture was concentrated under reduced pressure, and dichloromethane (10 ml) and water (5 ml) were added to the residue, and the mixture was extracted twice with dichloromethane. The organic layer was washed with 10% aqueous citric acid solution (10 ml) and then with 15% brine (10 ml), and dried over magnesium sulfate. The desiccant was filtered off, and the filtrate was concentrated under reduced pressure. The obtained residue was purified by silica gel column chromatography (gradient; hexane:ethyl acetate=9:1?1:1) to give Boc-DOPA-OMe (980 mg, 3.15 mmol, yield 76%) as a pale-peach candy-like substance. (0198) 1H-NMR (400 MHz,CDCl3) delta: 1.42 (s, 9H), 2.89-3.01 (m, 2H), 3.71 (s, 3H), 4.49-4.54 (m, 1H), 5.01 (d, 1H, J=8.0 Hz), 5.51 (s, 1H), 5.65 (s, 1H), 6.54 (dd, 1H, J=1.5, 8.0 Hz), 6.65 (br, 1H), 6.76 (d, 1H, J=8.1 Hz). (0199) ESIMS (m/z) : 310.0 ([M-H]-). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping