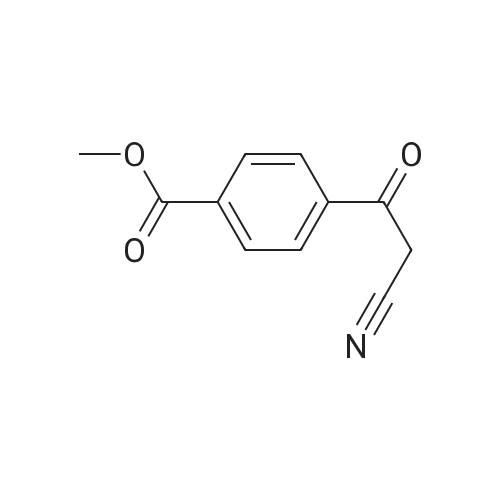
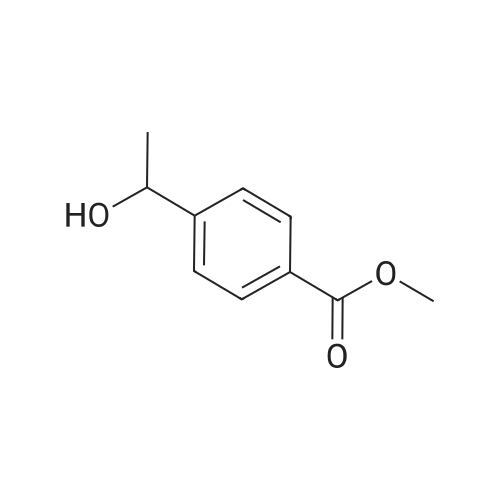
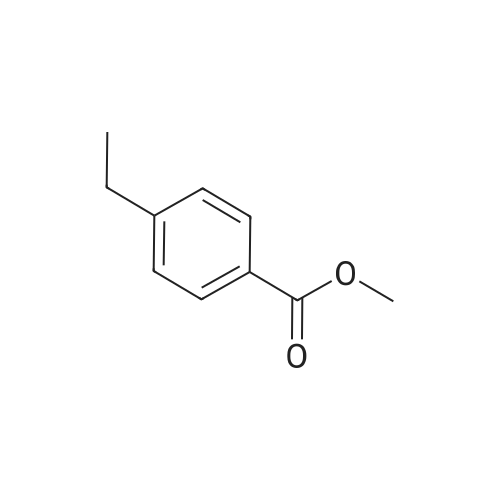
| 94% |
With sodium hypophosphite monohydrate; 5%-palladium/activated carbon; tetrabutyl-ammonium chloride; In 2-methyltetrahydrofuran; water; at 60℃; for 2.7h;Schlenk technique; |
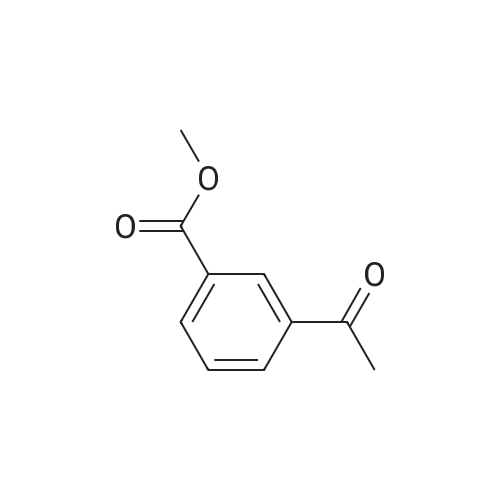
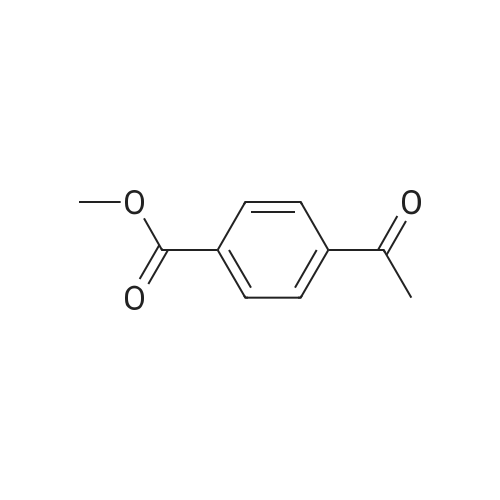
General procedure: In a Schlenk tube (10mL), a solution of ketone compound (1mmol), tetrabutylammonium chloride (20mg, 72mumol, 7mol%), and Pd/C 5% wt (50% in water) (55mg, 26mumol, 2.6mol%) in 2-MeTHF (1mL) was stirred at room temperature (20C) for 10-20min. To this mixture was added a solution of sodium hypophosphite monohydrate (424mg, 4mmol, 4equiv) in water (2.5mL). The reaction mixture was heated at 60C. After dilution in CH2Cl2 (10mL), water (10mL) was added. The aqueous phase was extracted with CH2Cl2 (2×20mL). The combined organic layers were dried (Na2SO4), filtered, and concentrated. Purification by flash chromatography on silica gel was performed for products 4a, 5a, 10a, 12a, 15a, and 19c. 4.2.1 4-(1-Hydroxyethyl)benzoic acid methyl ester [84851-56-9]11d (1a) (0021) Procedure A; 2.7h; colorless oil (170mg, 94%). 1H NMR (300MHz, CDCl3) delta (ppm)=1.51 (d, 3H, J=6.5Hz, CH3), 1.84 (brs, 1H, OH), 3.91 (s, 3H, OCH3), 4.97 (q, 1H, J=6.5Hz, CH-OH), 7.45 (d, 2H, J=8.3Hz, Harom), 8.02 (d, 2H, J=8.3Hz, Harom). |
| 85% |
With sodium tetrahydroborate; ethanol; at 0 - 20℃; for 12.5h; |
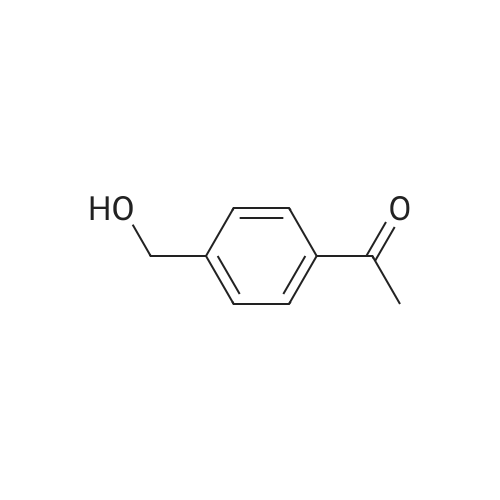
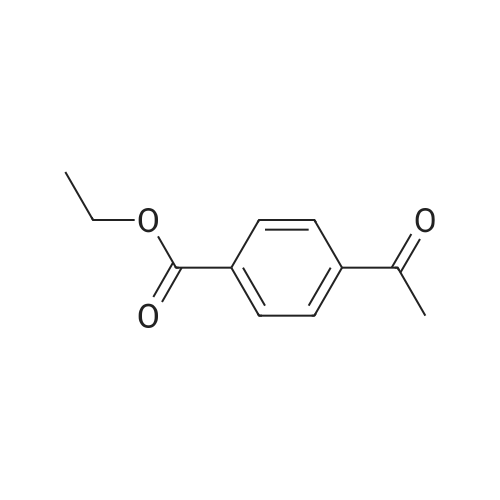
methyl 4-( I -hydroxy ethyl) benzoate. To a solution of methyl 4-acetylbenzoate (1.78 g, 10 mmol) in ethanol (100 mL) was added sodium borohydride (0.76 g, 20 mmol) in portions at 0C. The mixture was stirred for 30 minutes and warmed to 20C. Then the mixture was stirred at the same temperature for 12 hours. After that, the mixture was concentrated in vacuo to give methyl 4-(l -hydroxy ethyl) benzoate (1.54 g, 85%). |
| 85% |
With sodium tetrahydroborate; ethanol; at 0 - 20℃; for 12.5h; |
To a solution of methyl 4-acetylbenzoate (1.78 g, 10 mmol) in ethanol (100 mL) was added sodium borohydride (0.76 g, 20 mmol) in portions at 0 C. The mixture was stirred for 30 minutes and warmed to 20 C. Then the mixture was stirred at the same temperature for 12 hours. After that, the mixture was concentrated in vacuo to give methyl 4-(1-hydroxyethyl)benzoate (1.54 g, 85%). |
| 44.9% |
With methanol; sodium tetrahydroborate; at 20℃; for 0.25h; |
A solution of methyl 4-acetylbenzoate (220 mg, 0.8 mmol) in methanol was added(50 mL) was added sodium borohydride (60 mg, 1.6 mmol) and stirred at room temperature for 15 minutes.An appropriate amount of water was added to the system and extracted with ethyl acetate. The organic phase was dried over anhydrous sodium sulfate and concentrated to give the title compound 20 (100 mg, 44.9%). |
|
With sodium tetrahydroborate; ethanol; at 0℃; for 1h; |
To a stirred solution of methyl 4-acetylbenzoate (358 mg, 2.0 mmol) in EtOH (10 mL) was added NaBH4 (152 mg, 4 mmol) at 0 C. The resulting mixture was stirred at same temperature for 1 h. The excess solvent was removed under vacuum. And the residue was quenched with H20, and extracted with EtOAc (3 x 20 mL). The combined organic extracts were washed with brine (40 mL), dried over sodium sulfate, and concentrated under vacuum. The crude product was obtained as colorless oil (310 mg, 86%) and used directly into next step. 1H NMR (400 MHz, CDC13) delta 8.07 - 7.96 (m, 2H), 7.44 (d, J = 8.2 Hz, 2H), 5.04 - 4.87 (m, 1H), 3.91 (s, 3H), 1.50 (d, J = 6.5 Hz, 3H). 13C NMR (100 MHz, CDC13) delta 167.0, 150.9, 129.9 (2C), 129.2, 125.3 (2C), 70.0, 52.1, 25.3. |
| 81%Chromat. |
With C28H35ClCoN5(1+)*Cl(1-); potassium tert-butylate; hydrogen; In tetrahydrofuran; at 60℃; under 37503.8 Torr; for 16h;Autoclave; |
General procedure: In an argon filled glove box, the cobalt catalyst (LNHC/CoCl2 or Co-2a) and the base wereweighted into a 4mL vial equipped with a magnetic stir bar, followed by addition of the solvent.After shaking of the vial for 30 seconds, the carbonyl substrate was then added. The vial wasplaced into a Parr Instruments autoclave, which was then sealed, removed from the glove boxand purged with hydrogen gas. The autoclave was heated to certain temperature. After reactionfor 16 hours, the autoclave was cooled down to 0 oC before releasing the hydrogen gas. Forquantitative GC analysis, biphenyl (1.0 mmol) as internal standard was added. The organiclayer was then filtrated and diluted for GC analysis. The stereo-selectivity of the hydrogenatedproducts of cyclohexanones were determined by NMR with mesitylene as the internal standard.The desired hydrogenation product was further isolated by flash column chromatography. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping