Alternatived Products of [ 359012-63-8 ]
Product Details of [ 359012-63-8 ]
| CAS No. : | 359012-63-8 |
MDL No. : | MFCD16660041 |
| Formula : |
C16H11BO2
|
Boiling Point : |
No data available |
| Linear Structure Formula : | - |
InChI Key : | LDPCTBXVSGTSNJ-UHFFFAOYSA-N |
| M.W : |
246.07
|
Pubchem ID : | 23088535 |
| Synonyms : |
|
Safety of [ 359012-63-8 ]
Application In Synthesis of [ 359012-63-8 ]
* All experimental methods are cited from the reference, please refer to the original source for details. We do not guarantee the accuracy of the content in the reference.
- Downstream synthetic route of [ 359012-63-8 ]
- 1
-
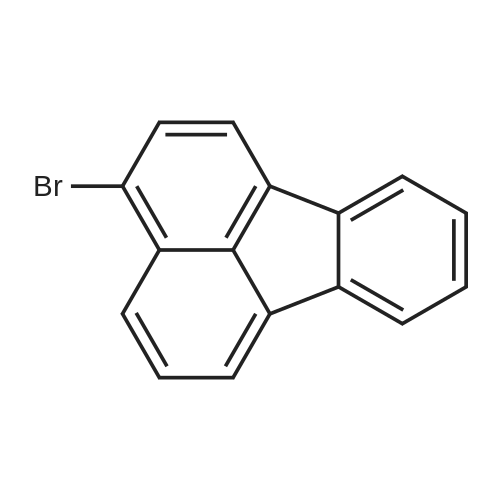 [ 13438-50-1 ]
[ 13438-50-1 ]

-
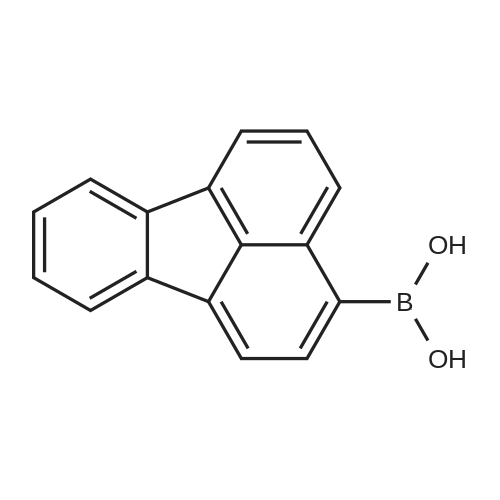 [ 359012-63-8 ]
[ 359012-63-8 ]
| Yield | Reaction Conditions | Operation in experiment |
| 65% |
|
Preparation of Compound 2-2 [104] 24.5 g (87.1 mmol) of Compound 2-1 was dissolved in 500 mL of THF, cooled to -78, added with 45 mL of n-BuLi (2.5 M in hexane), and then stirred for 1 hour. Subsequently, this mixture was added with 15 mL of B(OMe)3 and stirred for 2 hours, and the reaction was terminated with 250 mL of aqueous ammonium chloride. The mixture thus obtained was extracted with 1 L of EA and then washed with 200 mL of distilled water. The resultant organic layer was dried with anhydrous MgSO4, treated under reduced pressure to remove the organic solvent, and then recrystallized, thus obtaining Compound 2-2 (14 g, 65percent). |
|
|
Under the atmosphere of argon, 10.0 g of the crystals obtained above, 120 ml of dehydrated ether (manufactured by HIROSHIMA WAKO Co., Ltd.) and 120 ml of dehydrated toluene (manufactured by HIROSHIMA WAKO Co., Ltd.) were placed into a 500 ml flask, and the resultant mixture was cooled at -64° C. in a dry ice bath. To the cooled mixture, 25 ml of a 1.6 M hexane solution of butyllithium (manufactured by HIROSHIMA WAKO Co., Ltd.) was added dropwise over 30 minutes, and the reaction was allowed to proceed at -64° C. for 2 hours. To the resultant reaction mixture, 8 g of triisopropyl borate (manufactured by TOKYO KASEI Co., Ltd.) was added dropwise over 20 minutes. After the addition was completed, the temperature was adjusted at the room temperature, and the reaction mixture was stirred for 12 hours. After the resultant reaction mixture was cooled with ice, 100 ml of 2 N hydrochloric acid was added at a temperature of 10° C. or lower, and 25 ml of toluene was added. The organic phase separated from the resultant mixture was dried with sodium sulfate and concentrated under a reduced pressure. Hexane was added to the resultant solution, and formed crystals were separated by filtration. The obtained crystals were dissolved into 120 ml of tetrahydrofuran. To the resultant solution, 15 ml of concentrated hydrochloric acid and 0.15 g of tetrabutylammonium bromide were added, and the resultant mixture was stirred for 12 hours. The formed crystals were separated by filtration and dried, and 7.0 g of crystals of 3-fluorantheneboric acid were obtained. |
- 2
-
 [ 5419-55-6 ]
[ 5419-55-6 ]

-
 [ 13438-50-1 ]
[ 13438-50-1 ]

-
 [ 359012-63-8 ]
[ 359012-63-8 ]
| Yield | Reaction Conditions | Operation in experiment |
| 100% |
With n-butyllithium; water; In tetrahydrofuran; at -80℃; for 0.25h; |
5.6 g of <strong>[13438-50-1]3-bromofluoranthene</strong> (molecular weight 280, 0.02 mol) was dissolved in 80 ml of dry THF, A solution of n-butyllithium 9 ml (2.5 M, 0.0225 mol) was added dropwise at -80 ° C,Stirring for 15min, And 20 ml of triisopropyl borate was added dropwise. Hydrolysis, the pH was adjusted to neutral, acid derivative precipitated 4.95 g, a yield of almost 100percent |
| 70% |
|
In the nitrogen,Clean and dry 1000ml three-mouth bottle,Add 4a and THF in proportion,Cool down to -100°C,Lithium butyl lithium was added dropwise, and the insulation was kept for 2 hours.Triisopropyl borate was added dropwise and incubated for 4 h.Acidification of hydrochloric acid, washing, drying solvent, toluene beating, get 4b;HPLC: 99.5percent, yield: 70percent |
| 60% |
|
Preparation of compound 1-3 [97] After dissolving compound 1-2 (7.7 g, 27.5 mmol) in tetrahydrofuran (THF) (250 mL), the reaction mixture was cooled to -78°C. 2.5 M n-BuLi in hexane (17.6 mL, 44 mmol) was added to the reaction mixture, and the reaction mixture was stirred for 1 hour. B(Oi-Pr)3 (12.6 mL, 55 mmol) was added slowly at the same temperature, and the reaction mixture was stirred for 2 hours. After stirring, the reaction mixture was quenched with adding 2M HCl, was extracted with distilled water and EA, and the organic layer was concentrated. The organic layer was recrystallized with methylene chloride(MC) and hexane to obtain compound 1-3 (4.0 g, 60 percent). |
- 3
-
 [ 359012-63-8 ]
[ 359012-63-8 ]

-
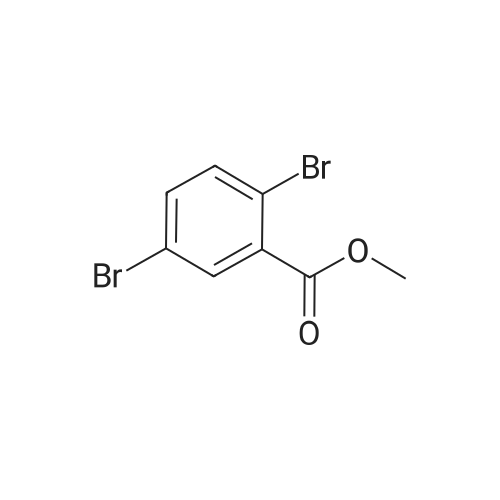 [ 57381-43-8 ]
[ 57381-43-8 ]

-
C24H15BrO2
[ No CAS ]
| Yield | Reaction Conditions | Operation in experiment |
| 87.68% |
With tetrakis(triphenylphosphine) palladium(0); potassium carbonate; In ethanol; water; toluene; for 8h;Inert atmosphere; Reflux; |
In a 1 L three-necked flask, fluoranthene-3-boronic acid (49.2 g, 0.20 mol) <strong>[57381-43-8]2,5-dibromobenzoic acid methyl ester</strong> (61.7 g, 0.21 mol) Potassium carbonate (55.2 g, 0.40 mol), 165 · 6g water, Pd (PPh3) 4 (1.156 g, 1 Ommol), Toluene (400 mL), Anhydrous ethanol (lOOmL), N2 to protect, heat to reflux, heat reaction 8 hours, stop reaction, cool to 25 ° C, liquid separation, collecting organic phase, washed to neutral, organic phase under pressure to remove the solvent, pure toluene column chromatography, toluene And recrystallized from absolute ethanol to give intermediate 1-1 (yield 87.68percent). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science















 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping








