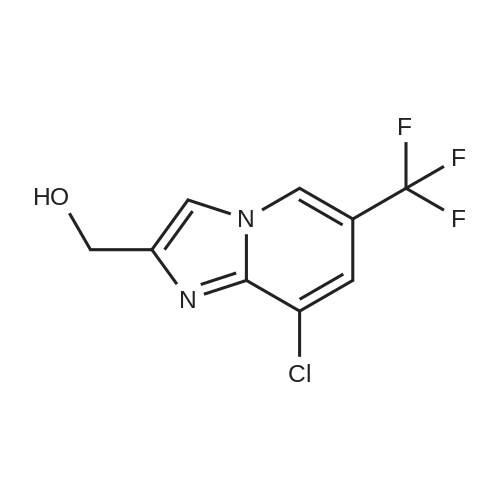
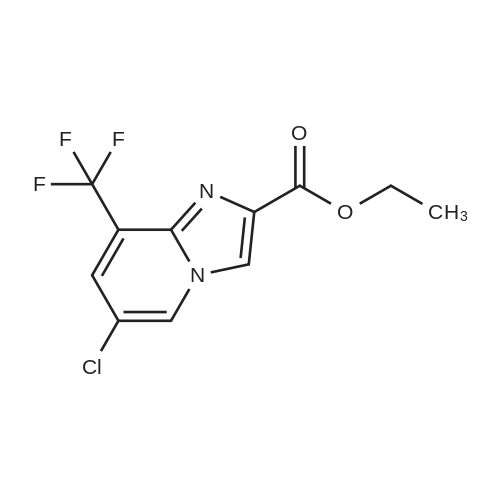
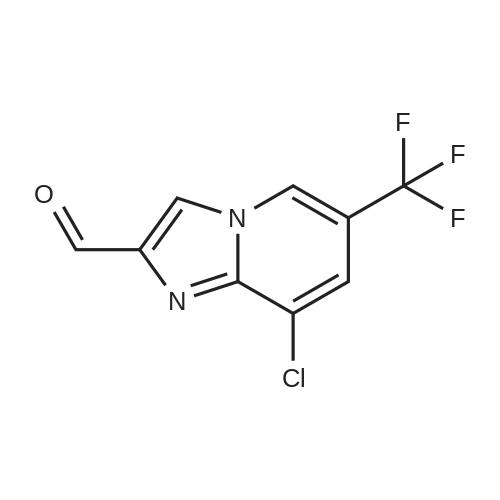
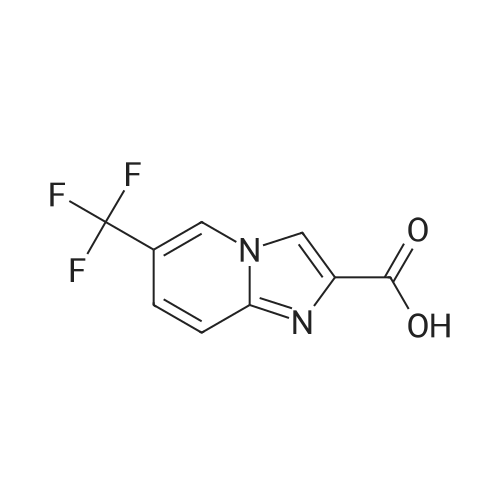
Alternatived Products of [ 353258-35-2 ]
Product Details of [ 353258-35-2 ]
| CAS No. : | 353258-35-2 |
MDL No. : | MFCD00831510 |
| Formula : |
C9H4ClF3N2O2
|
Boiling Point : |
No data available |
| Linear Structure Formula : | - |
InChI Key : | BNADDMQVCIRZBW-UHFFFAOYSA-N |
| M.W : |
264.59
|
Pubchem ID : | 3928817 |
| Synonyms : |
|
Safety of [ 353258-35-2 ]
Application In Synthesis of [ 353258-35-2 ]
* All experimental methods are cited from the reference, please refer to the original source for details. We do not guarantee the accuracy of the content in the reference.
- Downstream synthetic route of [ 353258-35-2 ]
- 1
-
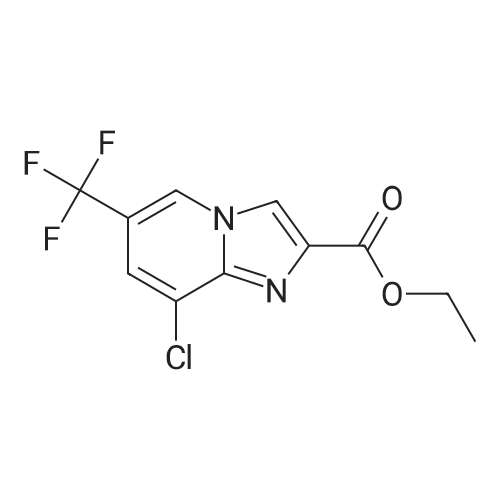 [ 353258-31-8 ]
[ 353258-31-8 ]

-
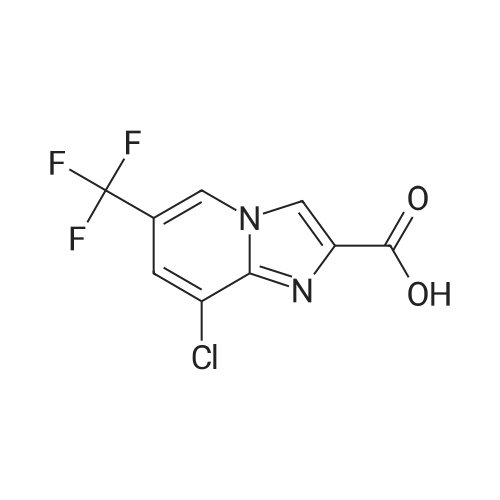 [ 353258-35-2 ]
[ 353258-35-2 ]
| Yield | Reaction Conditions | Operation in experiment |
| 86% |
With lithium hydroxide monohydrate; In ethanol;Reflux; |
Add 8-chloro-6-(trifluoromethyl)imidazo[1,2-A]pyridine-2-carboxylic acid ethyl ester 2.93 g to a 100 ml bottle(10mmol), add 50ml of ethanol, add 0.84g (20mmol) of lithium hydroxide monohydrate, reflux reaction, TLC tracking reactionAfter the end of the reaction, the pH was adjusted to 3, and a pale yellow precipitate was precipitated, washed with suction and dried to give 2.28 g, yield 86%. |
| 80% |
|
8-Chloro-6-(trifluoromethyl)-imidazo[l,2-α]pyridine-2-carboxylic acid (10).; To a stirred solution of ester 9 (10 g, 34 mmoL) in MeOH (100 mL) was added 1 M NaOH (100 mL). The mixture was heated to 500C for Ih. The reaction mixture was concentrated in vacuo. Water was added to the residue and the mixture acidified to pH 4 using acetic acid. The resulting precipitate was filtered, washed with water, and dried to afford intermediate 10 (7.2 g, 80% yield) as a white solid. |
| 77% |
With water; sodium hydroxide; In methanol; at 50℃; for 1.0h; |
To a stirred solution of ethyl 8-chloro-6-(trifluoromethyl)imidazo[ 1 ,2-a]pyridine-2- carboxylate (6) (64.0 g, 0.22 mol) in MeOH (64.0 mL) was added 1M aqueous NaOH (640.0 mL). The reaction mixture was heated at 50C for 1 h and then cooled to room temperature. The mixture was concentrated under vacuum. Water was added to the residue and the mixture was acidified to pH=4 with AcOH. The resulting precipitate was collected by filtration, washed with water and dried under vacuum to afford compound (7) (24.0 g) as an off-white solid. The filtrate was extracted with EtOAc and the combined organic layers were dried over anhydrous Na2SO4 and concentrated under vacuum to afford another portion of compound (7) (20.0 g) as an offwhite solid (combined yield 77%). |
| 77% |
With water; sodium hydroxide; In methanol; at 50℃; for 1.0h; |
[00254] To a stirred solution of ethyl 8-chloro-6-(trifluorom ethyl )imidazo[l, 2- ]pyridine-2- carboxylate (6) (64.0 g, 0.22 mol) in MeOH (64.0 mL) was added 1M aqueous NaOH (640.0 mL). The reaction mixture was heated at 50C for 1 h and then cooled to room temperature. The mixture was concentrated under vacuum. Water was added to the residue and the mixture was acidified to pH=4 with AcOH. The resulting precipitate was collected by filtration, washed with water and dried under vacuum to afford compound (7) (24.0 g) as an off-white solid. The filtrate was extracted with EtOAc and the combined organic layers were dried over anhydrous Na2S04 and concentrated under vacuum to afford another portion of compound (7) (20.0 g) as an off- white solid (combined yield 77%). |
|
|
To a solution of 2-amino-3-chloro-5-(trifluoromethyl)pyridine (24.7 g, 126 mmol) in 1 ,2-dimethoxyethane (260 mL) at 0 0C was added ethyl bromopyruvate (17.43 mL, 138 mmol) dropwise. The reaction mixture was warmed to room temperature and stirred for three days to form a suspension. The reaction mixture was then extracted with dichloromethane (2 x 200 mL) and washed with water (2 x 200 mL). The combined dichloromethane extracts were dried over magnesium sulfate and concentrated under reduced pressure to obtain a solid residue. The combined water washes were saturated with sodium carbonate, extracted with ethyl acetate (2 x 200 mL), and dried over magnesium sulfate. The combined ethyl acetate extracts and the residue obtained from the concentration of the dichloromethane were combined, and the mixture was concentrated under reduced pressure to obtain a solid.This solid was dissolved in ethanol (800 mL), and aqueous 50 % sodium hydroxide(40 g, 500 mmol) combined with additional water (150 mL) was added dropwise. The reaction mixture was stirred at room temperature overnight to form a suspension. The reaction mixture was acidified to pH 2 with concentrated hydrochloric acid and then cooled and stirred for several hours to precipitate a solid. The solid product was isolated by filtration using a glass-fritted filter funnel, washed with water, and air dried under a stream of air overnight to afford 13 g of the title compound as a solid. 1H NMR (CDCl3) δ 8.51 (s, IH), 8.40 (s, IH), 7.55 (s, IH). |
| 20.0 g |
With water; sodium hydroxide; In methanol; at 50℃; for 1.0h; |
To a stirred solution of ethyl 8-chloro-6-(trifluoromethyl)imidazo[ 1 ,2-a]pyridine-2- carboxylate (31) (64.0 g, 0.22 mol) in MeOH (64.0 mL) was added 1M aqueous NaOH (640.0 mL). The reaction mixture was heated at 50C for 1 h and then cooled to room temperature. The mixture was concentrated under vacuum. Water was added to the residue and the mixture was acidified to pH=4 with AcOH. The resulting precipitate was collected by filtration, washed with water and dried under vacuum to afford compound (32) (24.0 g) as an off-white solid. The filtrate was extracted with EtOAc and the combined organic layers were dried over anhydrous Na2 SO4 and concentrated under vacuum to afford another portion of compound (32) (20.0 g) as an off-white solid (combined yield 77%). |

Reference:
[1]Patent: CN108276352,2018,A .Location in patent: Paragraph 0368; 0369; 0370
[2]Patent: WO2010/65760,2010,A1 .Location in patent: Page/Page column 102
[3]Patent: WO2017/83756,2017,A1 .Location in patent: Paragraph 00212
[4]Patent: WO2018/211324,2018,A1 .Location in patent: Paragraph 00254
[5]Patent: WO2010/129500,2010,A2 .Location in patent: Page/Page column 56-57
[6]Bioorganic and Medicinal Chemistry Letters,2017,vol. 27,p. 1572 - 1575
[7]Patent: WO2018/211323,2018,A1 .Location in patent: Paragraph 0025

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science















 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping