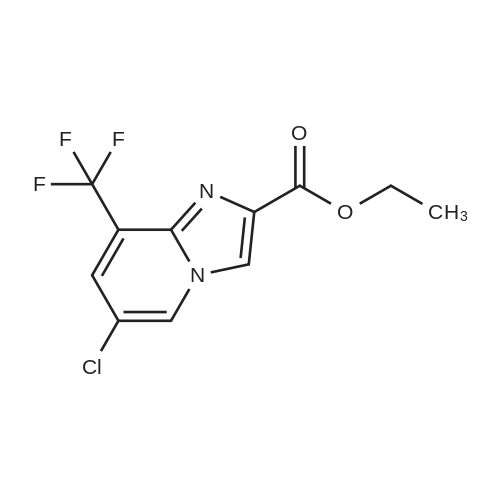
Alternatived Products of [ 353258-31-8 ]
Product Details of [ 353258-31-8 ]
| CAS No. : | 353258-31-8 |
MDL No. : | MFCD01833013 |
| Formula : |
C11H8ClF3N2O2
|
Boiling Point : |
- |
| Linear Structure Formula : | - |
InChI Key : | AKACORAIGDTIFR-UHFFFAOYSA-N |
| M.W : |
292.64
|
Pubchem ID : | 737427 |
| Synonyms : |
|
Safety of [ 353258-31-8 ]
Application In Synthesis of [ 353258-31-8 ]
* All experimental methods are cited from the reference, please refer to the original source for details. We do not guarantee the accuracy of the content in the reference.
- Downstream synthetic route of [ 353258-31-8 ]
- 1
-
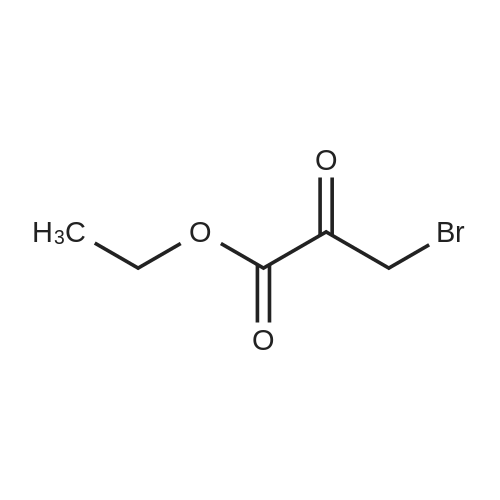 [ 70-23-5 ]
[ 70-23-5 ]

-
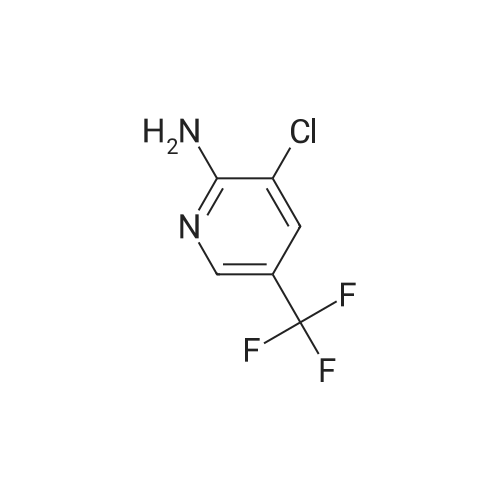 [ 79456-26-1 ]
[ 79456-26-1 ]

-
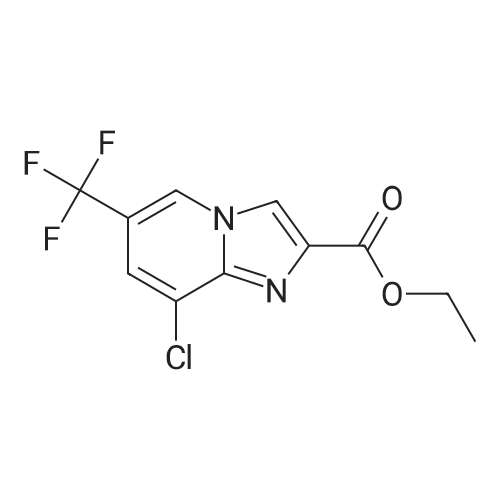 [ 353258-31-8 ]
[ 353258-31-8 ]
| Yield | Reaction Conditions | Operation in experiment |
| 93% |
In ethanol; at 20 - 80℃; for 12.0h; |
Intermediate 10; 8-Chloro-6-(trifluoromethyl)imidazo[l,2-fl]pyridine-2-carboxylic acid; Ethyl 8-chloro-6-(trifluoromethyl)-imidazo [ 1 ,2-a] pyridine-2-carboxylate (9).; To a stirred solution of 2-amino-3-chloro-5-trifluoromethyl pyridine 7 (12.5 g, 63.6 mmol) in EtOH (125 rnL) was added ethyl bromopyruvate 8 (20 mL, 159 mmol) at room temperature. The resulting mixture was heated to 80 0C for 12 h. The reaction mixture was cooled to ambient temperature and concentrated. The residue was suspended in diethyl ether and the resulting solid was filtered and dried under vacuum to afford 9 (17.3g, 93% yield) as a light yellow solid. |
| 86% |
In ethanol; at 80℃; for 48.0h; |
To a stirred solution of 2-amino-3-chloro-5-trifluoromethylpyridine (5) (50.0 g, 0.25 mmol) in EtOH (500 mL) was added ethylbromopyruvate (80.0 mL,0.64 mol) at room temperature. The reaction mixture was heated at 80C for 48 h and then cooled to room temperature. The mixture was concentrated and the residue was suspended in diethyl ether. The resulting precipitate was collected by filtration and dried under vacuum to afford ethyl 8-chloro- 6-(trifluoromethyl)imidazo[1,2-a]pyridine-2-carboxylate (6) (64.0 g, 86%) as an off-white solid. |
| 86% |
In ethanol; at 80℃; for 48.0h; |
[00253] To a stirred solution of 2-amino-3-chloro-5-trifluoromethylpyridine (5) (50.0 g, 0.25 mmol) in EtOH (500 mL) was added ethylbromopyruvate (80.0 mL,0.64 mol) at room temperature. The reaction mixture was heated at 80C for 48 h and then cooled to room temperature. The mixture was concentrated and the residue was suspended in diethyl ether. The resulting precipitate was collected by filtration and dried under vacuum to afford ethyl 8-chloro- 6-(trifluoromethyl)imidazo[l,2- ]pyridine-2-carboxylate (6) (64.0 g, 86%) as an off-white solid. |
| 86% |
In ethanol; at 80℃; for 48.0h; |
To a stirred solution of 2-amino-3-chloro-5-trifluoromethylpyridine (30) (50.0 g, 0.25 mmol) in EtOH (500 mL) was added ethylbromopyruvate (80.0 mL, 0.64 mol) at room temperature. The reaction mixture was heated at 80C for 48 h and then cooled to room temperature. The mixture was concentrated and the residue was suspended in diethyl ether. The resulting precipitate was collected by filtration and dried under vacuum to afford ethyl 8-chloro- 6-(trifluoromethyl)imidazo[ 1 ,2-a]pyridine-2-carboxylate (31) (64.0 g, 86%) as an off-white solid. |
| 75% |
In tetrahydrofuran;Reflux; |
2-amino-3-chloro-5-trifluoromethylpyridine 3.92 g (20 mmol) was added to a 250 ml bottle.Ethyl 3-bromopyruvate3.9 g (20 mmol), 100 ml of tetrahydrofuran, and heated to reflux.TLC followed the progress of the reaction, and after the reaction was completed, the solvent was distilled off under reduced pressure.A saturated sodium chloride solution (60 mL) was added to the residue and dichloromethane (50 mL×3)The organic phases were combined, dried over anhydrous sodium sulfate, concentrated and dried.The white solid product was obtained 4.4 g, yield 75%. |
|
In 1,2-dimethoxyethane; at 0 - 25℃; for 72.0h; |
To a solution of 2-amino-3-chloro-5-(trifluoromethyl)pyridine (24.7 g, 126 mmol) in 1 ,2-dimethoxyethane (260 mL) at 0 0C was added ethyl bromopyruvate (17.43 mL, 138 mmol) dropwise. The reaction mixture was warmed to room temperature and stirred for three days to form a suspension. The reaction mixture was then extracted with dichloromethane (2 x 200 mL) and washed with water (2 x 200 mL). The combined dichloromethane extracts were dried over magnesium sulfate and concentrated under reduced pressure to obtain a solid residue. The combined water washes were saturated with sodium carbonate, extracted with ethyl acetate (2 x 200 mL), and dried over magnesium sulfate. The combined ethyl acetate extracts and the residue obtained from the concentration of the dichloromethane were combined, and the mixture was concentrated under reduced pressure to obtain a solid.This solid was dissolved in ethanol (800 mL), and aqueous 50 % sodium hydroxide(40 g, 500 mmol) combined with additional water (150 mL) was added dropwise. The reaction mixture was stirred at room temperature overnight to form a suspension. The reaction mixture was acidified to pH 2 with concentrated hydrochloric acid and then cooled and stirred for several hours to precipitate a solid. The solid product was isolated by filtration using a glass-fritted filter funnel, washed with water, and air dried under a stream of air overnight to afford 13 g of the title compound as a solid. 1H NMR (CDCl3) δ 8.51 (s, IH), 8.40 (s, IH), 7.55 (s, IH). |

Reference:
[1]Patent: WO2010/65760,2010,A1 .Location in patent: Page/Page column 102
[2]Patent: WO2017/83756,2017,A1 .Location in patent: Paragraph 00211
[3]Patent: WO2018/211324,2018,A1 .Location in patent: Paragraph 00253
[4]Patent: WO2018/211323,2018,A1 .Location in patent: Paragraph 00251
[5]Patent: CN108276352,2018,A .Location in patent: Paragraph 0365; 0366; 0367
[6]Patent: WO2010/129500,2010,A2 .Location in patent: Page/Page column 56-57
[7]Bioorganic and Medicinal Chemistry Letters,2017,vol. 27,p. 1572 - 1575
- 2
-
 [ 353258-31-8 ]
[ 353258-31-8 ]

-
 [ 1228376-01-9 ]
[ 1228376-01-9 ]
| Yield | Reaction Conditions | Operation in experiment |
| 88.88% |
With ammonium hydroxide; In 1,4-dioxane; at 60℃; for 4.0h;sealed tube; |
Intermediate 83; 8-Chloro-7V'-hydroxy-6-(trifluoromethyl)imidazo[l,2-fl]pyridine-2-carboximidamide; S-Chloro--CtrifluoromethylJimidazotl^-αjpyridine-l-carboxamide (171).; To a stirred solution of 9 (5.0 g, 17.12 mmol), prepared as described in Intermediate 10, in dioxane (30 rnL) was added NH4OH (60 mL) and the reaction was stirred at 60 0C for 4 h in a sealed tube. Solvent was removed and the residue obtained was crystallize from EtOAc, filtered and dried to obtain 171 (4 g, 88.88%). |
- 3
-
 [ 353258-31-8 ]
[ 353258-31-8 ]

-
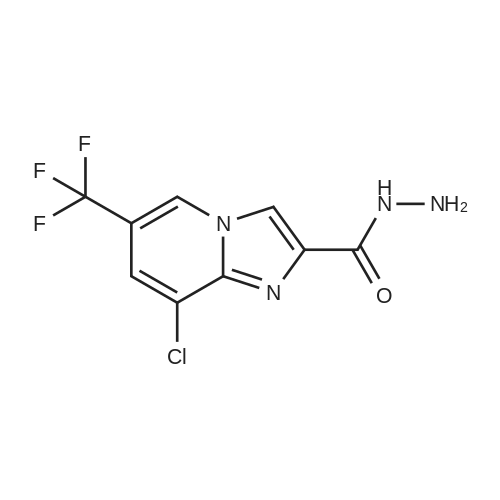 [ 883032-94-8 ]
[ 883032-94-8 ]
| Yield | Reaction Conditions | Operation in experiment |
| 49% |
With hydrazine hydrate; In ethanol; for 3.0h;Reflux; |
Example 18; 2,5-Dichloro-4-(5-(8-chloro-6-(trifluoromethyl)imidazo [ 1 ,2-α] pyridin-2-yl)- 1 ,3,4-thiadiazol-2-yl)phenol; 8-Chloro-6-(trifluoromethyl) imidazo [1, 2-a] pyridine-2-carbohydrazide (87).; To a stirred solution of ester 9 (15 g, 51 mmol), prepared as described in the synthesis of Intermediate 10, in EtOH (100 mL) was added hydrazine hydrate (7.7 g, 150 mmol). The reaction mixture was stirred at reflux for 3 h, after which, the reaction mixture was concentrated in vacuo. To the resulting residue, water was added and the mixture extracted with EtOAc. The organic layer was washed with water, saturated NaCl solution, dried over Na2SO4 and concentrated in vacuo to afford intermediate 87 (7.0 g, yield 49%) as a white solid. |
|
With hydrazine; In ethanol; water; for 16.0h;Reflux; |
1-107: To a soln of ethyl 8-chloro-6-(trifluoromethyl)imidazo[l,2-a]pyridine-2- carboxylate (3.00 g, 10.3 mmol) in EtOH (20 mL) was added hydrazine (35% in H20, 4.6 mL, 51 mmol) at RT. The soln was heated under reflux for 16 h. The soln was then cooled down and water was added. The soln was extracted with EtOAc and the combined organics were dried over MgS04, filtered, and concentrated to afford compound 1-107. |
- 4
-
 [ 353258-31-8 ]
[ 353258-31-8 ]

-
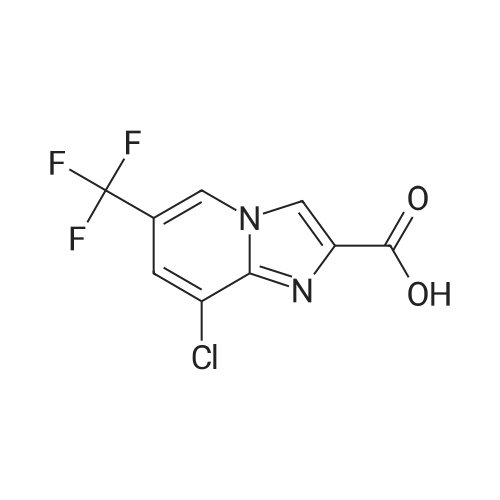 [ 353258-35-2 ]
[ 353258-35-2 ]
| Yield | Reaction Conditions | Operation in experiment |
| 86% |
With lithium hydroxide monohydrate; In ethanol;Reflux; |
Add 8-chloro-6-(trifluoromethyl)imidazo[1,2-A]pyridine-2-carboxylic acid ethyl ester 2.93 g to a 100 ml bottle(10mmol), add 50ml of ethanol, add 0.84g (20mmol) of lithium hydroxide monohydrate, reflux reaction, TLC tracking reactionAfter the end of the reaction, the pH was adjusted to 3, and a pale yellow precipitate was precipitated, washed with suction and dried to give 2.28 g, yield 86%. |
| 80% |
|
8-Chloro-6-(trifluoromethyl)-imidazo[l,2-α]pyridine-2-carboxylic acid (10).; To a stirred solution of ester 9 (10 g, 34 mmoL) in MeOH (100 mL) was added 1 M NaOH (100 mL). The mixture was heated to 500C for Ih. The reaction mixture was concentrated in vacuo. Water was added to the residue and the mixture acidified to pH 4 using acetic acid. The resulting precipitate was filtered, washed with water, and dried to afford intermediate 10 (7.2 g, 80% yield) as a white solid. |
| 77% |
With water; sodium hydroxide; In methanol; at 50℃; for 1.0h; |
To a stirred solution of ethyl 8-chloro-6-(trifluoromethyl)imidazo[ 1 ,2-a]pyridine-2- carboxylate (6) (64.0 g, 0.22 mol) in MeOH (64.0 mL) was added 1M aqueous NaOH (640.0 mL). The reaction mixture was heated at 50C for 1 h and then cooled to room temperature. The mixture was concentrated under vacuum. Water was added to the residue and the mixture was acidified to pH=4 with AcOH. The resulting precipitate was collected by filtration, washed with water and dried under vacuum to afford compound (7) (24.0 g) as an off-white solid. The filtrate was extracted with EtOAc and the combined organic layers were dried over anhydrous Na2SO4 and concentrated under vacuum to afford another portion of compound (7) (20.0 g) as an offwhite solid (combined yield 77%). |
| 77% |
With water; sodium hydroxide; In methanol; at 50℃; for 1.0h; |
[00254] To a stirred solution of ethyl 8-chloro-6-(trifluorom ethyl )imidazo[l, 2- ]pyridine-2- carboxylate (6) (64.0 g, 0.22 mol) in MeOH (64.0 mL) was added 1M aqueous NaOH (640.0 mL). The reaction mixture was heated at 50C for 1 h and then cooled to room temperature. The mixture was concentrated under vacuum. Water was added to the residue and the mixture was acidified to pH=4 with AcOH. The resulting precipitate was collected by filtration, washed with water and dried under vacuum to afford compound (7) (24.0 g) as an off-white solid. The filtrate was extracted with EtOAc and the combined organic layers were dried over anhydrous Na2S04 and concentrated under vacuum to afford another portion of compound (7) (20.0 g) as an off- white solid (combined yield 77%). |
|
|
To a solution of 2-amino-3-chloro-5-(trifluoromethyl)pyridine (24.7 g, 126 mmol) in 1 ,2-dimethoxyethane (260 mL) at 0 0C was added ethyl bromopyruvate (17.43 mL, 138 mmol) dropwise. The reaction mixture was warmed to room temperature and stirred for three days to form a suspension. The reaction mixture was then extracted with dichloromethane (2 x 200 mL) and washed with water (2 x 200 mL). The combined dichloromethane extracts were dried over magnesium sulfate and concentrated under reduced pressure to obtain a solid residue. The combined water washes were saturated with sodium carbonate, extracted with ethyl acetate (2 x 200 mL), and dried over magnesium sulfate. The combined ethyl acetate extracts and the residue obtained from the concentration of the dichloromethane were combined, and the mixture was concentrated under reduced pressure to obtain a solid.This solid was dissolved in ethanol (800 mL), and aqueous 50 % sodium hydroxide(40 g, 500 mmol) combined with additional water (150 mL) was added dropwise. The reaction mixture was stirred at room temperature overnight to form a suspension. The reaction mixture was acidified to pH 2 with concentrated hydrochloric acid and then cooled and stirred for several hours to precipitate a solid. The solid product was isolated by filtration using a glass-fritted filter funnel, washed with water, and air dried under a stream of air overnight to afford 13 g of the title compound as a solid. 1H NMR (CDCl3) δ 8.51 (s, IH), 8.40 (s, IH), 7.55 (s, IH). |
| 20.0 g |
With water; sodium hydroxide; In methanol; at 50℃; for 1.0h; |
To a stirred solution of ethyl 8-chloro-6-(trifluoromethyl)imidazo[ 1 ,2-a]pyridine-2- carboxylate (31) (64.0 g, 0.22 mol) in MeOH (64.0 mL) was added 1M aqueous NaOH (640.0 mL). The reaction mixture was heated at 50C for 1 h and then cooled to room temperature. The mixture was concentrated under vacuum. Water was added to the residue and the mixture was acidified to pH=4 with AcOH. The resulting precipitate was collected by filtration, washed with water and dried under vacuum to afford compound (32) (24.0 g) as an off-white solid. The filtrate was extracted with EtOAc and the combined organic layers were dried over anhydrous Na2 SO4 and concentrated under vacuum to afford another portion of compound (32) (20.0 g) as an off-white solid (combined yield 77%). |

Reference:
[1]Patent: CN108276352,2018,A .Location in patent: Paragraph 0368; 0369; 0370
[2]Patent: WO2010/65760,2010,A1 .Location in patent: Page/Page column 102
[3]Patent: WO2017/83756,2017,A1 .Location in patent: Paragraph 00212
[4]Patent: WO2018/211324,2018,A1 .Location in patent: Paragraph 00254
[5]Patent: WO2010/129500,2010,A2 .Location in patent: Page/Page column 56-57
[6]Bioorganic and Medicinal Chemistry Letters,2017,vol. 27,p. 1572 - 1575
[7]Patent: WO2018/211323,2018,A1 .Location in patent: Paragraph 0025
- 5
-
 [ 353258-31-8 ]
[ 353258-31-8 ]

-
2-chloro-5-methoxy-benzene sulfonamide
[ No CAS ]

-
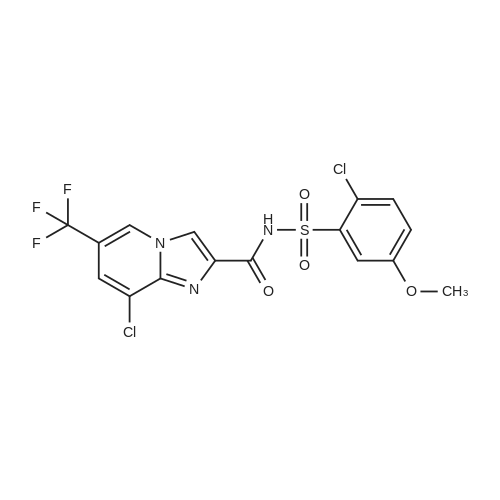 [ 1254304-22-7 ]
[ 1254304-22-7 ]
| Yield | Reaction Conditions | Operation in experiment |
| 89.6% |
With diethylaluminium chloride; In 1,2-dichloro-ethane; at 23 - 75℃; for 1.5h;Inert atmosphere; |
To a stirred slurry of 8-chloro-6-(trifluoromethyl)imidazo[l,2,a]pyridine-2-carboxylic acid ethyl ester (see PCT Patent Publication WO 2010/129500 for preparation) (8 g, 27.3 mmol) and 2-chloro-5-methoxybenzenesulfonamide (see PCT Patent Publication WO 2010/129500 for preparation) (6.4 g, 28.7 mmol, 1.05 equiv) in 1 ,2-dichloroethane (41 mL) at 22 C under nitrogen was added diethylaluminum chloride (4 mL, 31.9 mmol, 1.18 equiv) over approximately 5 minutes. The addition of diethylaluminum chloride was accompanied by about 23 C temperature rise and moderate foaming. After complete addition of diethylaluminum chloride the temperature of the reaction mixture was adjusted to 75 C. The reaction mixture was held with efficient stirring for about 1.5 h at 75 C during which it became an off-white slurry. After about 1.5 h, HPLC analysis indicated <1 area % of 8- chloro-6-(trifluoromethyl)imidazo[l,2,a]pyridine-2-carboxylic acid ethyl ester remaining. Aqueous 10% acetic acid (41 mL, 68.3 mmol, 2.5 equiv) was then added resulting in some frothing and the reaction mass transformed to a thick slurry and then to a clear biphasic solution. The reaction mass was then cooled down to 45 C and the two phases separated. The organic phase was then transferred to a jacketed-reactor together with additional 1 ,2- dichloroethane (20 mL) that was used to dissolve some precipitated solids. The organic phase was heated to distill 1 ,2-dichloroethane under atmospheric pressure. After collection of about 30 ml of the distillate, acetic acid (17 mL) was added to the jacketed-reactor and distillation continued. When the reactor's temperature reached 102 C, water (64 mL) was added to it and distillation continued. After collection of another 30 mL of distillate, water (about 40 mL) was added to the reactor, the distillation was ended and the batch brought down to room temperature. The batch was then warmed to 75 C and held stirring for about 5.5h. The batch was then cooled to room temperature and then filtered. The filter cake was washed with water (20 ml). The solid product was split into two portions, the larger portion was dried in a vacuum oven at 80 C for 16 hours and the smaller portion was air-dried for 16 hours to give the title compound (9.98 g and 1.48g respectively) in 89.6% combined yield and with purity = 96.7 a% (by HPLC). Both the air-dried and the vacuum-dried product conformed with polymorph Form A. The polymorph Form A was characterized by its powder X-ray diffraction pattern (See Characterization Example 2). |
- 6
-
 [ 353258-31-8 ]
[ 353258-31-8 ]

-
C28H27Cl2F4N5O4S
[ No CAS ]
- 7
-
 [ 353258-31-8 ]
[ 353258-31-8 ]

-
(R)-2-amino-4-{5-chloro-4-[5-(8-chloro-6-trifluoromethylimidazo[1,2-a]pyridin-2-yl)-[1,3,4]-thiadiazol-2-yl]-2-fluoro-phenoxy}butan-1-ol hydrochloride
[ No CAS ]
- 8
-
 [ 353258-31-8 ]
[ 353258-31-8 ]

-
(R)-2-amino-4-{5-chloro-4-[5-(8-chloro-6-trifluoromethylimidazo[1,2-a]pyridin-2-yl)-[1,3,4]-thiadiazol-2-yl]-2-fluoro-phenoxy}butan-1-ol trifluoroacetate
[ No CAS ]
- 9
-
 [ 353258-31-8 ]
[ 353258-31-8 ]

-
C16H7Cl2F5N4O2
[ No CAS ]
- 10
-
 [ 353258-31-8 ]
[ 353258-31-8 ]

-
C16H5Cl2F5N4S
[ No CAS ]
- 11
-
 [ 353258-31-8 ]
[ 353258-31-8 ]

-
 [ 1254304-22-7 ]
[ 1254304-22-7 ]
- 12
-
 [ 353258-31-8 ]
[ 353258-31-8 ]

-
 [ 1254305-48-0 ]
[ 1254305-48-0 ]
- 13
-
 [ 353258-31-8 ]
[ 353258-31-8 ]

-
C15H7Cl3F3N3O3S
[ No CAS ]
- 14
-
 [ 353258-31-8 ]
[ 353258-31-8 ]

-
 [ 1254305-47-9 ]
[ 1254305-47-9 ]

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science















 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping