|
|
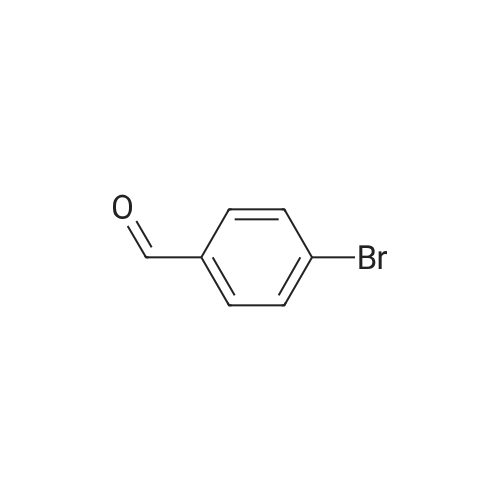
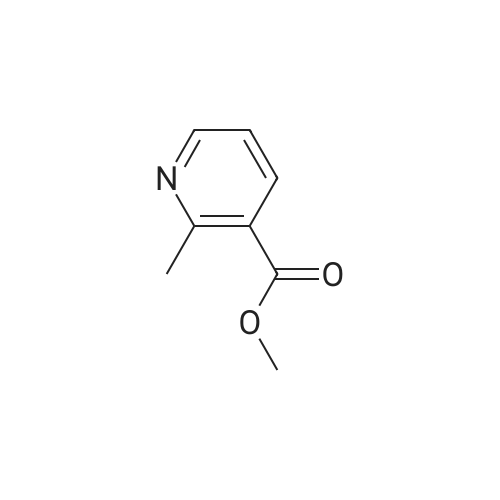
Example 9; Step 1: 2-f(£yZ)-2-(4-bromophenyl)vinyl1-3-carboxy-5-chloropyridinium chloride; Potassium tert-butoxide (IM solution in THF, 60 mL, 60 mmol) was added to a solution of 4-bromobenzaldehyde (5.6 g, 30 mmol) and methyl 5-chloro~2-methylnicotinate (Marcoux, J.-F.; Marcotte, F.-A.; Wu, J.; Dormer, P.G.; Davies, I. W.; Hughes, D.; Reider, PJ. J. Org, Chem. 2001, 66, 4194-4199) (5.6 g, 30 mmol) in 200 mL THF at O0C. The mixture was allowed to warm to ambient temperature and stirred for 12 hours. The reaction slurry was concentrated to give yellow/orange solids, then 50 mL of water and 50 mL of 6N HCl were added. After stirring the resulting slurry for 30 minutes, 200 mL of EtOH was added and the slurry was stirred for 4 hours. The slurry was filtered and dried to afford the title compound. 1H NMR (600 MHz, DMSO-D6) delta 8.76 (d, IH); 8.22 (d, IH); 8.02 (d, IH);7.79 (d, IH); 7.60-7.54 (m, 4H). LRMS (ESI) calc'd for C14H10BrClNO2 [M+H]+, 338.0; found 337.9. |
|
|
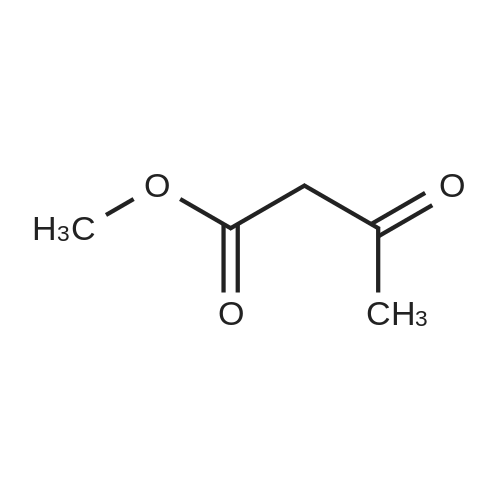
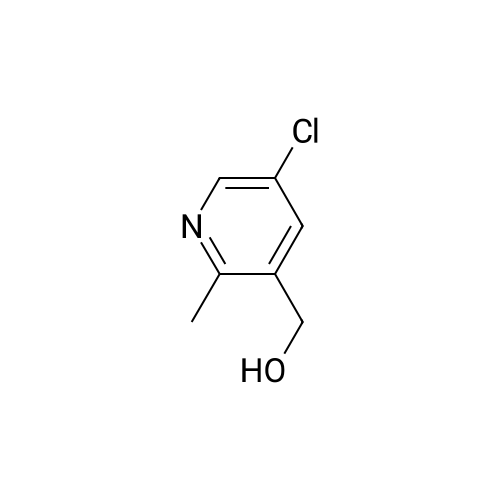
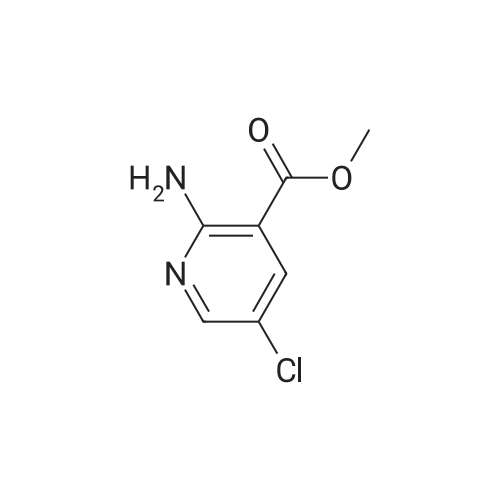
Potassium tert-butoxide (1 M solution in THF, 60 mL, 60 mmol) was added to a solution of 4-bromobenzaldehyde (5.6 g, 30 mmol) and <strong>[350597-49-8]methyl 5-chloro-2-methylnicotinate</strong> (Marcoux, J.-F.; Marcotte, F.-A.; Wu, J.; Dormer, P. G.; Davies, I. W.; Hughes, D.; Reider, P. J. J. Org. Chem. 2001, 66, 4194-4199) (5.6 g, 30 mmol) in 200 mL THF at 0 C. The mixture was allowed to warm to ambient temperature and stirred for 12 hours. The reaction slurry was concentrated to give yellow/orange solids, then 50 mL of water and 50 mL of 6N HCl were added. After stirring the resulting slurry for 30 minutes, 200 mL of EtOH was added and the slurry was stirred for 4 hours. The slurry was filtered and dried to afford the title compound. 1H NMR (600 MHz, DMSO-D6) delta 8.76 (d, 1H); 8.22 (d, 1H); 8.02 (d, 1H); 7.79 (d, 1H); 7.60-7.54 (m, 4H). LRMS (APCI) calculated for C14H10BrClNO2 [M+H]+, 338.0; found 337.9. |
|
|
Example 1; Step 1: 2-[(E/Z)-2-(4-bromophenyl)vinyl]-3-carboxy-5-chloropyridinium chloride; Potassium tert-butoxide (1M solution in THF, 60 mL, 60 mmol) was added to a solution of 4-bromobenzaldehyde (5.6 g, 30 mmol) and <strong>[350597-49-8]methyl 5-chloro-2-methylnicotinate</strong> (Marcoux, J.-F.; Marcotte, F.-A.; Wu, J.; Dormer, P. G.; Davies, I. W.; Hughes, D.; Reider, P. J. J. Org. Chem. 2001, 66, 4194-4199) (5.6 g, 30 mmol) in 200 mL THF at 0 C. The mixture was allowed to warm to ambient temperature and stirred for 12 hours. The reaction slurry was concentrated to give yellow/orange solids, then 50 mL of water and 50 mL of 6N HCl were added. After stirring the resulting slurry for 30 minutes, 200 mL of EtOH was added and the slurry was stirred for 4 hours. The slurry was filtered and dried to afford the title compound. 1H NMR (600 MHz, DMSO-D6) delta 8.76 (d, 1H); 8.22 (d, 1H); 8.02 (d, 1H); 7.79 (d, 1H); 7.60-7.54 (m, 4H). LRMS (APCI) calculated for C14H10BrClNO2 [M+H]+, 338.0; found 337.9. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping