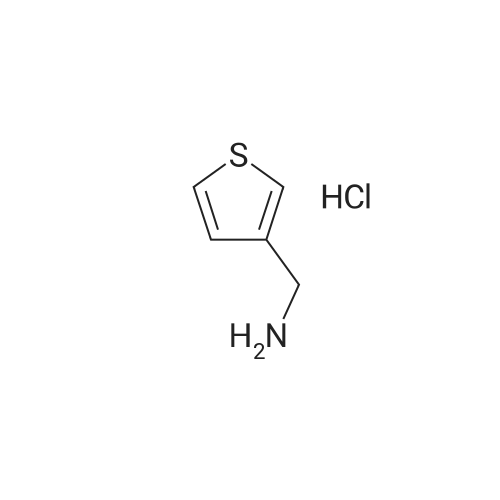
Alternatived Products of [ 34843-84-0 ]
Product Details of [ 34843-84-0 ]
| CAS No. : | 34843-84-0 |
MDL No. : | MFCD09265521 |
| Formula : |
C6H10ClNS
|
Boiling Point : |
- |
| Linear Structure Formula : | - |
InChI Key : | PYUCPHDVJORSMA-UHFFFAOYSA-N |
| M.W : |
163.67
|
Pubchem ID : | 12258738 |
| Synonyms : |
|
Application In Synthesis of [ 34843-84-0 ]
* All experimental methods are cited from the reference, please refer to the original source for details. We do not guarantee the accuracy of the content in the reference.
- Downstream synthetic route of [ 34843-84-0 ]
- 1
-
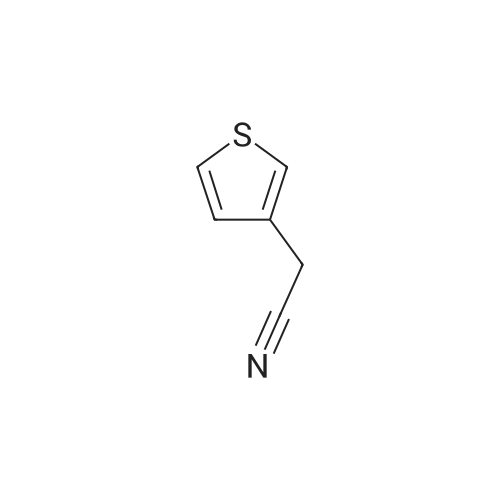 [ 13781-53-8 ]
[ 13781-53-8 ]

-
 [ 34843-84-0 ]
[ 34843-84-0 ]
| Yield | Reaction Conditions | Operation in experiment |
| 94% |
|
Thiophen-3-yl-acetonitrile (2.0 g, 16.2 mmol)Soluble in tetrahydrofuran (36mL),The borane-dimethyl sulfide complex (3.0 mL, 30.4 mmol) was slowly added, and the reaction mixture was heated to reflux for 16 hours. At room temperature, the reaction was quenched by the slow addition of methanol.Then add saturated hydrogen chloride / methanol solution (10mL),Stir at room temperature for 20 minutes, concentrate to give a solid initial product.Wash with diethyl ether (20 mL).The compound 41A was obtained as a white solid (2.5 g, yield: 94%). |
| 94% |
|
Preparation 1; 2-Thiophene-3-yl-ethylamine hydrochloride; Slowly add borane methyl sulfide complex (30.4 mL, 304.4 mmol) to a solution of thiophen-3-yl-acetonitrile (25.0 g, 203.0 mmol) in tetrahydrofuran (450 mL). Heat the reaction at reflux for 16 h and then cool to RT. Slowly quench the reaction with methanol (50 mL) until no foaming is observed. To this mixture slowly add methanol (100 mL) which is saturated with hydrogen chloride. Stir the mixture at RT for 20 min before concentrating in vacuo. Add methanol (100 mL) to the mixture and concentrate in vacuo. Suspend the resulting solid in diethyl ether (200 mL) and filter to afford 31.1 g (94%) of the crude title compound. MS/ES m/z 128.3 [M+H]+. |
| 94% |
|
Step 1. 2-Thiophene-3-yl-ethylamine hydrochloride; Slowly add borane methyl sulfide complex (30.4 mL, 304.4 mmol) to a solution of thiophen-3-yl-acetonitrile (25.0 g, 203.0 mmol) in tetrahydrofuran (450 mL). Heat the reaction at reflux for 16 h and then cool to RT. Slowly quench the reaction with methanol (50 mL) until no foaming is observed. To this mixture slowly add methanol (100 mL) which is saturated with hydrogen chloride. Stir the mixture at RT for 20 min before concentrating in vacuo. Add methanol (100 mL) to the mixture and concentrate in vacuo. Suspend the resulting solid in diethyl ether (200 mL) and filter to afford 31.1 g (94%) of the crude title compound. MS/ES m/z 128.3 [M+H]+ |
|
|
2-Thiophen-3-yl-ethylamine hydrochloride.A soln. of 3-thiopheneacetonitrile (18.44 g, 0.15 mol) in THF (245 mL) was added dropwise to a RT soln. of 1 M borane.THF complex (300 mL, 0.3 mol) under argon and the resulting mixture was heated to reflux for 3h followed by stirring at RT overnight. The reaction mixture was cautiously quenched with MeOH and subsequently concentrated in vacuo. The residue was dissolved in MeOH (150 mL) and 4M HCI in dioxane (105 mL, 2.8 eq.) was added cautiously under vigorous stirring and the mixture was stirred for 30 min. The soln. was evaporated in vacuo and the residue was re-dissolved in MeOH and re-evaporated. This dissolution and evaporation procedure was repeated a further two times to give the crude product as its HCI salt that was suspended in 3:7 'PrOH:EtOAc and stirred for 15 min. The suspension was filtered and the residue was dried in vacuo to give the title compound as a white solid (20.73 g). LC-MS A: tR = 0.33 min; [M+H+MeCN]+ = 169.06. |

Reference:
[1]Patent: CN108250128,2018,A .Location in patent: Paragraph 0698; 0700; 0701; 0702
[2]Patent: WO2007/146759,2007,A2 .Location in patent: Page/Page column 24
[3]Patent: WO2007/146758,2007,A2 .Location in patent: Page/Page column 33
[4]Patent: WO2007/146758,2007,A2 .Location in patent: Page/Page column 33
[5]Journal of Medicinal Chemistry,2014,vol. 57,p. 3687 - 3706
[6]Patent: WO2012/114252,2012,A1 .Location in patent: Page/Page column 46

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science















 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping