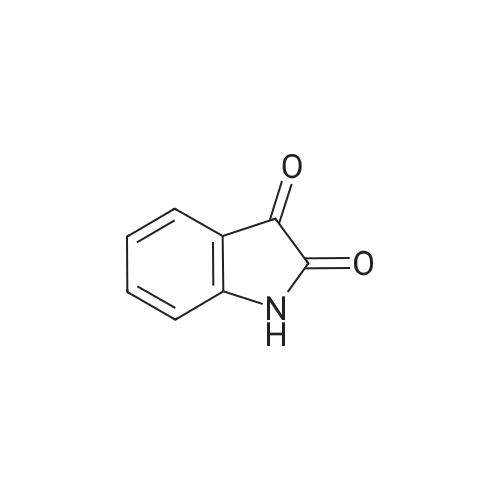
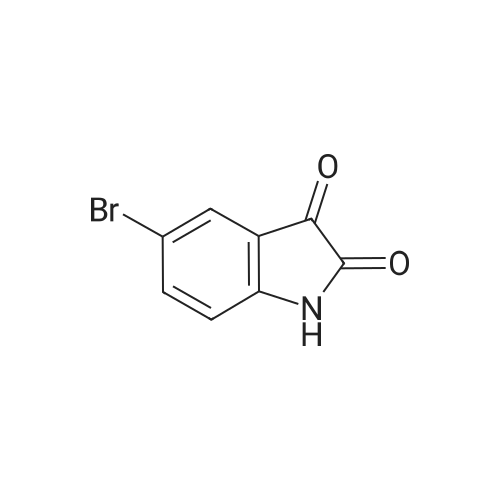
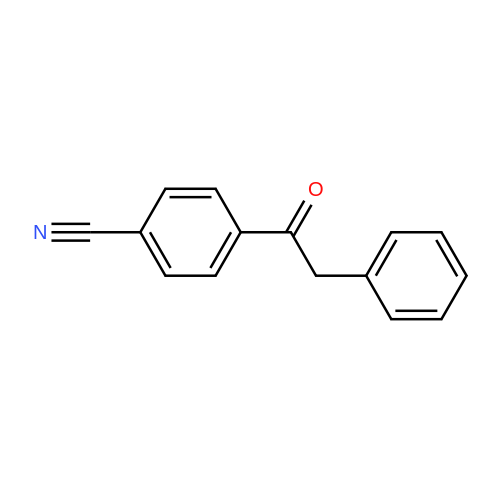
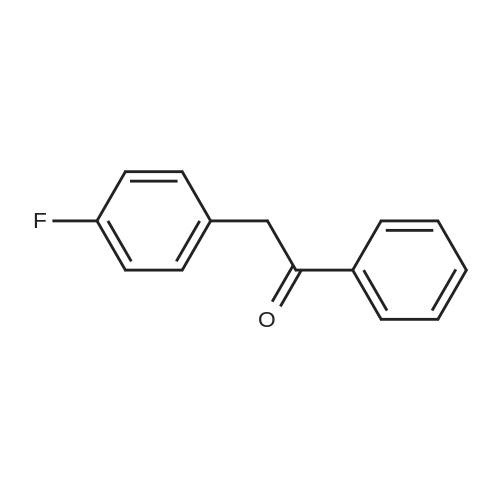
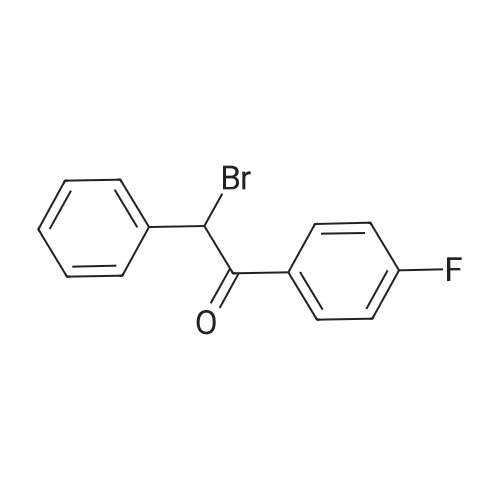
| 92% |
With dihydrogen peroxide; acetic acid; potassium bromide; In dichloromethane; water; at 0 - 5℃; for 2h; |
The bromination reaction using the bromide prepared above, specifically, 8.7 g of 4'-fluorophenyl-2-phenylethanone,80 mL of dichloromethane and 10 mL of water were added to a 250 mL three-necked flask, and then 8.6 g of the above-mentioned dried potassium bromide-containing solid was added.Stir and lower the temperature to 0-5 C, then add 3 mL of acetic acid dropwise. After the addition is completed, continue to maintain the temperature of 0-5 C and add 30% hydrogen peroxide 2.44 g.After the completion of the dropwise addition, the reaction was kept for 2 hours, and then liquid separation was carried out.18 mL of a 5% aqueous solution of sodium sulfite was added dropwise to the organic phase, and the mixture was stirred for 1 hour, and then separated.The organic phase was washed once with 18 mL of 5% aqueous sodium sulfite solution and then with 18 mL of 5% carbon.The aqueous sodium hydrogencarbonate solution and the 18 mL saturated saline solution were each washed once.The organic phase was dried over sodium sulfate, concentrated by filtration and solidified to give 10.9 g of pale yellow solid.2-bromo-1-(4'-fluorophenyl)-2-phenylethanone (Compound I), yield 92%, HPLC purity97.4%. |
| 90% |
With hydrogen bromide; dihydrogen peroxide; acetic acid; at 40℃; for 16h; |
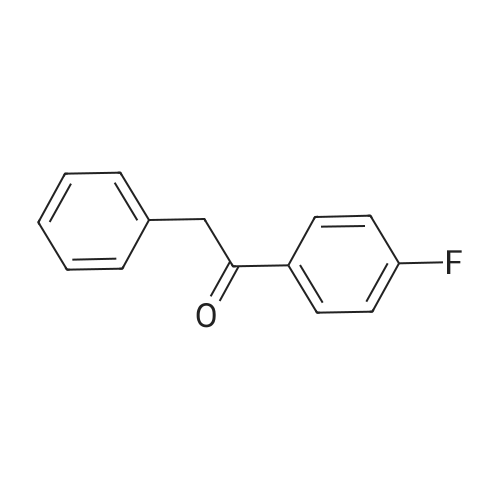
The third step, 10.7g 4-fluorophenylacetophenone was dissolved in 100ml of glacial acetic acid, 40% hydrobromic acid was added 15ml, stirred,Slowly dropping 30% mass fraction of hydrogen peroxide 9ml, 40 reaction 16h, TLC trace showed the end of the reaction.Unreacted bromine was removed by adding saturated aqueous sodium sulfite to the reaction mixture.The reaction mixture was extracted with 200 ml of ethyl acetate and an appropriate amount of aqueous sodium carbonate. The organic layer was separated and the organic layer was washed twice with aqueous sodium carbonate solution and dried over anhydrous magnesium sulfate.After filtration and spin drying, 13.25 g of 2-bromo-1- (4-fluorophenyl) -acetophenone was obtained as a yellow thick liquid in a yield of 90%. |
| 88% |
With copper(ll) bromide; In dichloromethane; ethyl acetate; for 18h;Reflux; Inert atmosphere; |
General procedure: Copper(II) bromide (CuBr2, 268 mg, 1.2 mmol) was added to a solution of skeleton 5 (1.0 mmol) in the co-solvent of EtOAc and CH2Cl2 (1:1, 20 mL), at 25 C. The reaction mixture was stirred at reflux for 18 h. The reaction mixture was cooled to 25 C. Saturated NaHCO3 (5 mL) was added to the reaction mixture and the solvent was concentrated. The residue was diluted with water (10 mL) and the mixture was extracted with EtOAc (3 x 20 mL). The combined organic layers were washed with brine, dried, filtered and evaporated to afford crude product. Purification on silica gel (hexanes/EtOAc = 10/1-6/1) afforded skeletons 3 and 6. |
| 85% |
|
Compound 4 (5.00 g, 23.34 mmol) was suspended in water (15 mL) in a flaskcovered with aluminum foil. Five drops of 40 % aqueous solution of HBr was added.The mixture was stirred at room temperature for 5 min, Br2 (2.05 g, 12.84 mmol) wasadded dropwise. The reaction mixture was stirred at room temperature for 5 h, and30 % aqueous solution of H2O2 (6.5 mL, 25.70 mmol) was slowly added. After 12 h,dichloromethane (30 mL) was added and the organic layer was washed with 5 %aqueous sodium sulfite (10 mL) and 5 % aqueous sodium chloride (2×20 mL) andthen dried over anhydrous magnesium sulfate. The organic mixture was concentrated to give a light yellow oil 5 (5.82 g, 85 % yield). 1H NMR (600 MHz, CDCl3) delta: 8.01-7.98 (m,2H), 7.49 (d, J=7.2 Hz,2H), 7.36-7.30 (m,3H), 7.07 (t, J=8.6 Hz,2H), 6.32 (s,1H). 13C NMR (150 MHz, CDCl3) delta: 189.33, 166.29, 164.59,135.52, 131.69, 131.63, 130.04, 128.89, 128.74, 115.71, 115.57, 51.07. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping