Alternatived Products of [ 33797-51-2 ]
Product Details of [ 33797-51-2 ]
| CAS No. : | 33797-51-2 |
MDL No. : | MFCD00011810 |
| Formula : |
C3H8IN
|
Boiling Point : |
- |
| Linear Structure Formula : | - |
InChI Key : | VVDUZZGYBOWDSQ-UHFFFAOYSA-M |
| M.W : |
185.01
|
Pubchem ID : | 2724133 |
| Synonyms : |
|
Application In Synthesis of [ 33797-51-2 ]
* All experimental methods are cited from the reference, please refer to the original source for details. We do not guarantee the accuracy of the content in the reference.
- Downstream synthetic route of [ 33797-51-2 ]
- 1
-
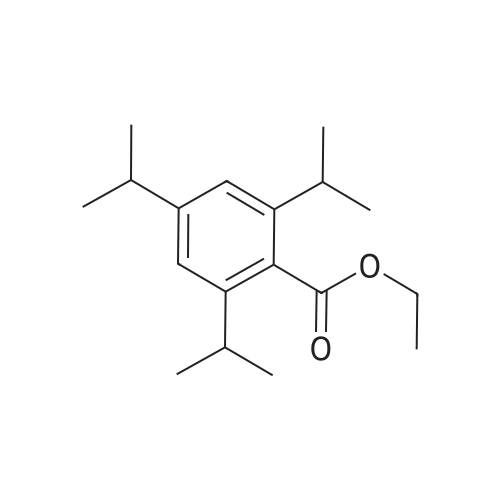 [ 63846-76-4 ]
[ 63846-76-4 ]

-
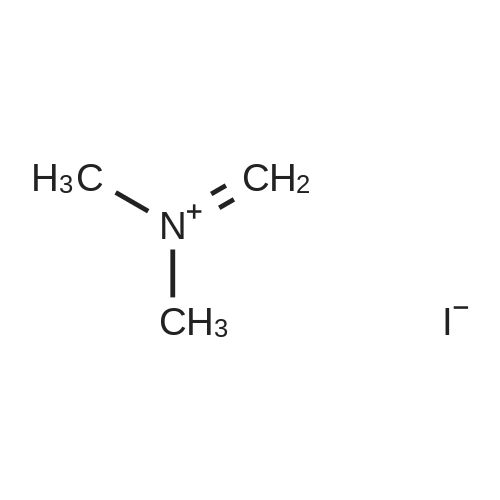 [ 33797-51-2 ]
[ 33797-51-2 ]

-
 [ 77256-53-2 ]
[ 77256-53-2 ]
- 2
-
 [ 33797-51-2 ]
[ 33797-51-2 ]

-
 [ 81971-99-5 ]
[ 81971-99-5 ]

-
3-[(4Z,10Z,15Z,19Z)-8-Dimethylaminomethyl-18-(2-methoxycarbonyl-ethyl)-3,7,12,17-tetramethyl-22,24-dihydro-porphin-2-yl]-propionic acid methyl ester
[ No CAS ]

-
3,8-bis<(dimethylamino)methyl>-13,17-bis<2-(methoxycarbonyl)ethyl>-2,7,12,18-tetramethylporphin
[ No CAS ]
- 3
-
 [ 33797-51-2 ]
[ 33797-51-2 ]

-
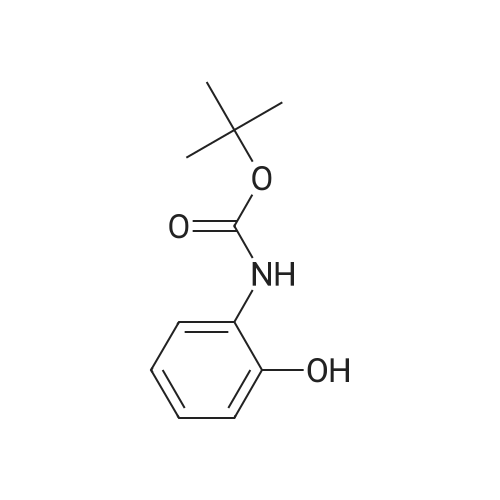 [ 186663-74-1 ]
[ 186663-74-1 ]

-
tert-butyl-3-((dimethylamino)methyl)-2-hydroxyphenylcarbamate
[ No CAS ]
- 4
-
 [ 33797-51-2 ]
[ 33797-51-2 ]

-
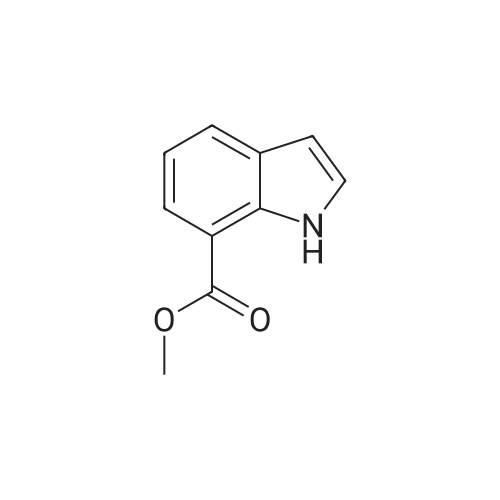 [ 93247-78-0 ]
[ 93247-78-0 ]

-
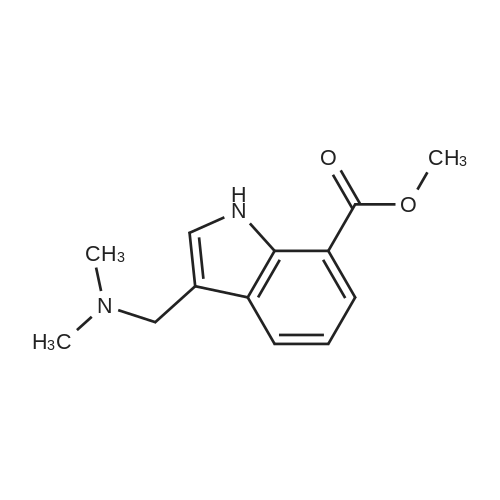 [ 312915-01-8 ]
[ 312915-01-8 ]
| Yield | Reaction Conditions | Operation in experiment |
|
|
lH-Indole-7-carboxylic acid, methyl ester (0.091 mol), Eschenmoser's salt (0.1 mol) in acetic acid (300 ml) were heated at 650C for 2 hours. The precipitate was filtered off, dissolved in DCM and potassium carbonate 10percent. Potassium carbonate (solid) was added and the mixture was stirred at room temperature for 1 hour and then extracted. The organic layer was separated, dried over MgSO4, filtered and the solvent was evaporated, yielding 1Og of intermediate 5. |
- 5
-
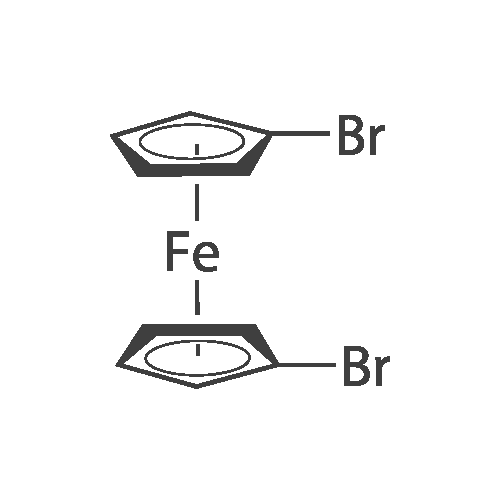 [ 1293-65-8 ]
[ 1293-65-8 ]

-
 [ 33797-51-2 ]
[ 33797-51-2 ]

-
 [ 259827-20-8 ]
[ 259827-20-8 ]
| Yield | Reaction Conditions | Operation in experiment |
| 87.5% |
|
Under a nitrogen atmosphere,2.0370 g (5.924 mmol) of <strong>[1293-65-8]1,1'-<strong>[1293-65-8]dibromoferrocene</strong></strong> was dissolved in THF (50 ml)Under cooling at -78 C.,4.0 ml (6.516 mmol) of n-butyllithium (1.6 M, n-hexane solution) was added and the mixture was stirred for 20 minutes.ThenN, N-dimethylmethylene ammonium iodide2.2 g (11.848 mmol) was added, then the mixture was allowed to stand at room temperature and stirred for 16 hours.An ammonium chloride aqueous solution (100 ml) and chloroform (150 ml) were added to the reaction solution, and the mixture was separated. The aqueous phase was further extracted twice with 50 ml of chloroform.The obtained organic phase (250 ml) was washed with saturated brine,After drying over sodium sulfate, concentration under reduced pressure gave a crude product. This was purified by silica gel column chromatography to obtain 1.6692 g (5.1835 mmol, yield 87.5%) of 1-bromo-1 '- ((dimethylamino) methyl) ferrocene as an intermediate. |
Categories

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping












