|
With phosphorus(V) oxybromide; In acetonitrile; at 20.0℃;Reflux; |
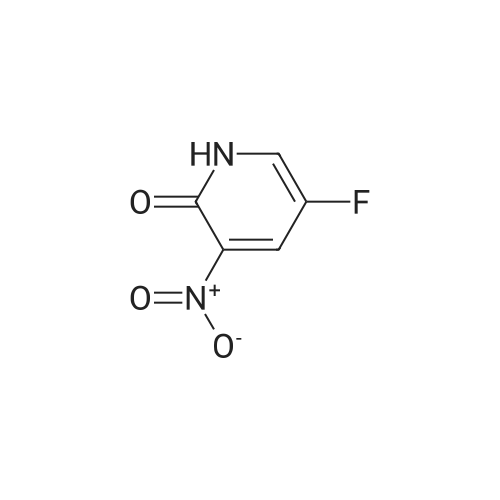
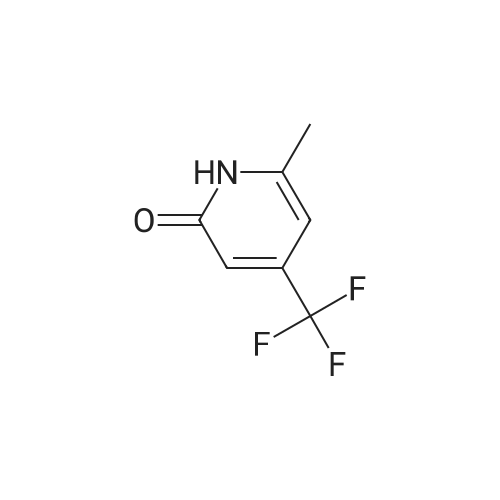
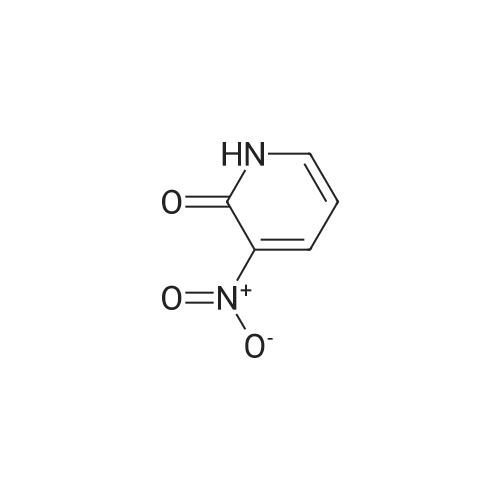
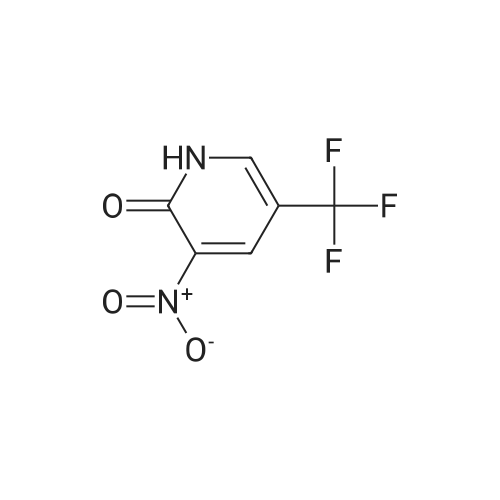
3-Nitro-5-(trifluoromethyl)pyridin-2-ol (31.00 g, 149 mmol) was dissolved in acetonitrile (250 ml) to give a dark brown solution. Phosphorus(V) oxybromide (85 g, 298 mmol) was added and the mixture was heated at reflux for 4 hours and then stirred at RT overnight. The reaction mixture was quenched by pouring into vigorously stirring water (600 ml) containing sodium hydrogencarbonate (1 10 g). The dark brown mixture was extracted with DCM (3 x 200 ml) and the organic phase was washed with water (200 ml) and brine (100ml), dried (MgS04) and concentrated under reduced pressure to afford the title product as a brown oil. H-NMR: [400MHz, CDCI3, ? 8.87 (1 H, d, J = 1.4Hz, ArH), 8.39 (1 H, d, J = 1 .9Hz, ArH). |
|
With phosphorus(V) oxybromide; at 20.0℃;Reflux; |
3-Nitro-5-(trifluoromethyl)pyridin-2-ol (31.00 g, 149 mmol) was dissolved in acetonitrile (250 ml) to give a dark brown solution. Phosphorus(V) oxybromide (85 g, 298 mmol) was added and the mixture was heated at reflux for 4 hours and then stirred at RT overnight. The reaction mixture was quenched by pouring into vigorously stirring water (600 ml) containing sodium hydrogencarbonate (1 10 g). The dark brown mixture was extracted with DCM (3 x 200 ml) and the organic phase was washed with water (200 ml) and brine (100ml), dried (MgS04) and concentrated under reduced pressure to afford the title product as a brown oil. H-NMR: [400MHz, CDCI3, ? 8.87 (1 H, d, J = 1.4Hz, ArH), 8.39 (1 H, d, J = 1.9Hz, ArH). |
|
With phosphorus(V) oxybromide; In acetonitrile; at 20.0℃;Reflux; |
3-Nitro-5-(trifluoromethyl)pyridin-2-ol (31 .00 g, 149 mmol) was dissolved in acetonitrile (250 ml) to give a dark brown solution. Phosphorus(V) oxybromide (85 g, 298 mmol) was added and the mixture was heated at reflux for 4 hours and then stirred at RT overnight. The reaction mixture was quenched by pouring into vigorously stirring water (600 ml) containing sodium hydrogencarbonate (1 10g). The dark brown mixture was extracted with DCM (3 x 200 ml) and the organic phase was washed with water (200 ml) and brine (100 ml), dried ( MgS04) and concentrated in vacuo to afford the title product as a brown oil. 1H-NMR: [400MHz, CDCI3, deltaEta 8.87 (1 H, d, J = 1.4Hz, ArH), 8.39 (1 H, d, J = 1.9Hz, ArH). |
| 2.44g |
With phosphorus(V) oxybromide; In acetonitrile; for 16.0h;Reflux; |
The 3-nitro-5-trifluoro-Methylpyridin-2-ol (q) (3.12g, 15mmol) in acetonitrile (25 ml) was added to solution POBr 3 (8.6g, 30mmol). Refluxed for 4 hours after stirring at room temperature for 12 hours. The reaction solution is poured into the rapidly stirred sodium bicarbonate (11g) aqueous solution (60 ml). Extraction with methylene chloride mixture (3x20ml), organic phase water (20 ml) and saturated sodium chloride solution (10 ml) after washing, drying by anhydrous sodium sulfate, concentrated to obtain the product (r) (2.44g, 9 . 0mmol) |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping