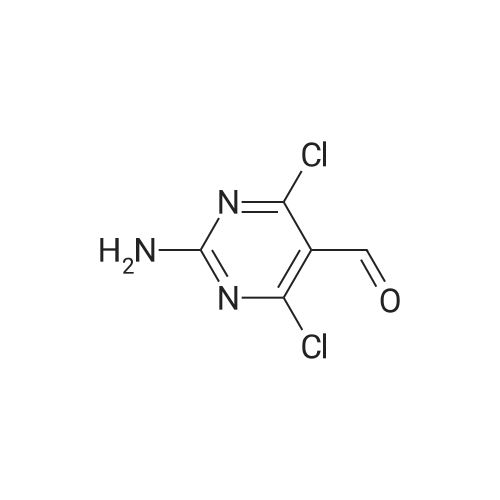
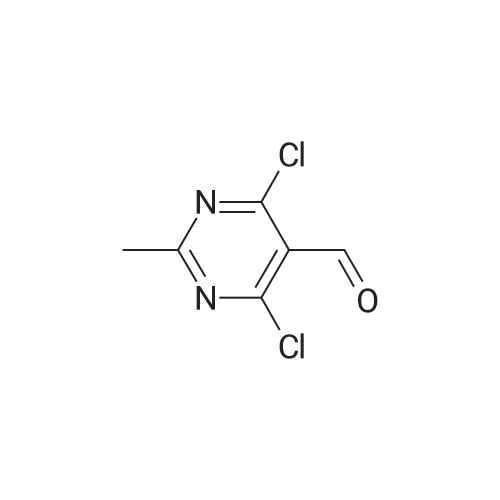
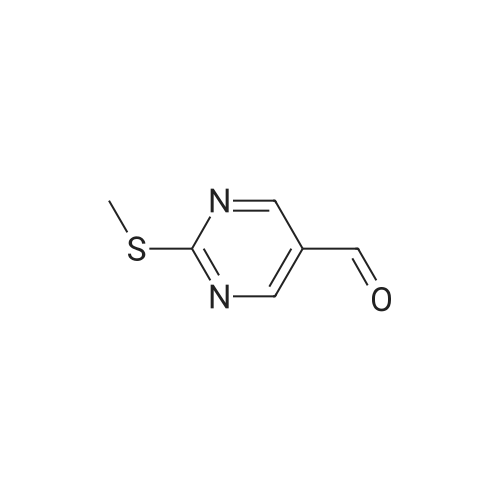
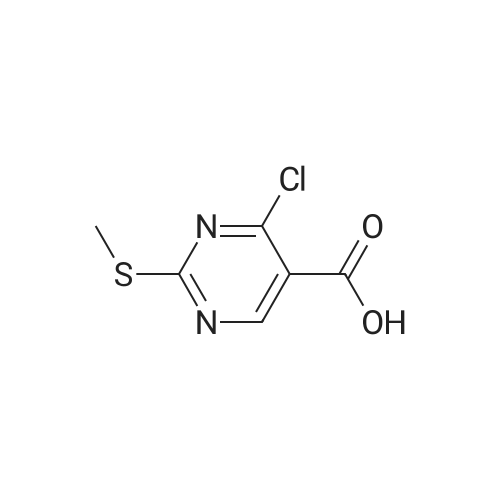
| 95% |
With hydrazine hydrate; triethylamine; In tetrahydrofuran; at 5℃; for 1h; |
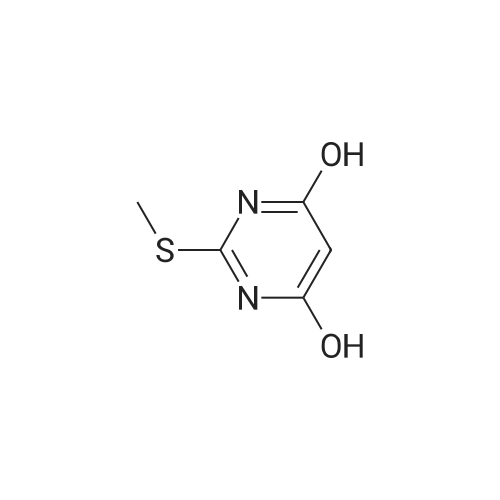
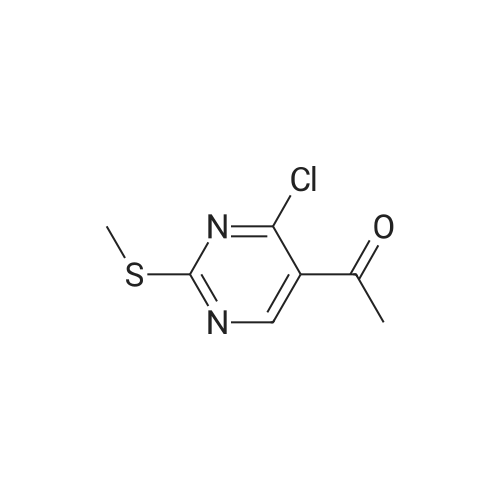
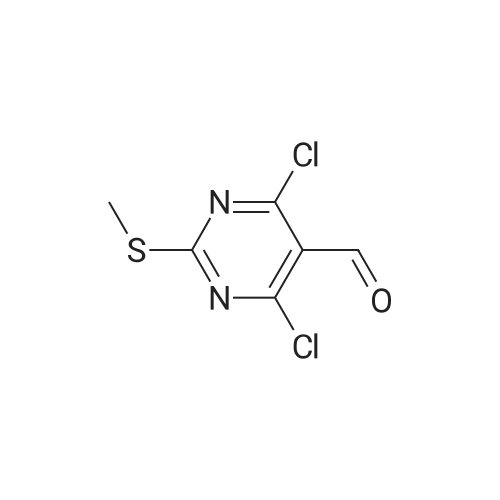
To a suspension of (27i) (2.5 g, 11.2 mmol) in THF (25 mL) cooled at 5C on an ice bath, were added dropwise hydrazine monohydrate (0.65 mL, 13 mmol) and triethylamine (1.8 mL, 13 mmol). After 1 hour stirring at 5C, the mixture was evaporated to dryness under vacuum and the residue was purified by silica gel column chromatography.Yield: 95%.Melting point: >300C.H NMR (DMSO -d6) d 2.59 (s, 3H, S CH3), 8.32 (s, 1H, CH), 14.25 (bs, 1H, N H).13C NMR (DMSO -d6) d 13.9 (SCH3), 109.6 (C-3a), 133.1 (C-3), 152.9-155.4 (C-4/C-7a), 168.7 (C- 6). |
|
With N-ethyl-N,N-diisopropylamine; hydrazine; In 1,4-dioxane; at 0℃; for 1088h;Heating / reflux;Product distribution / selectivity; |
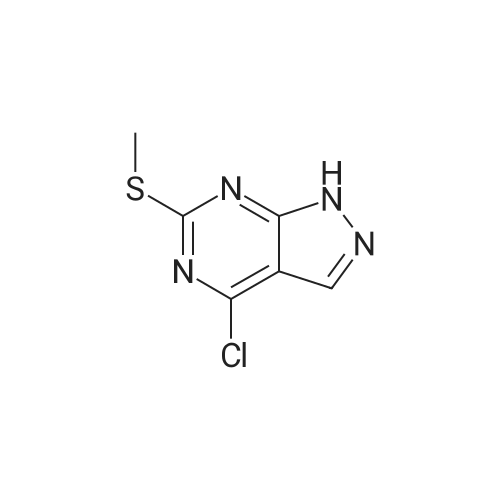
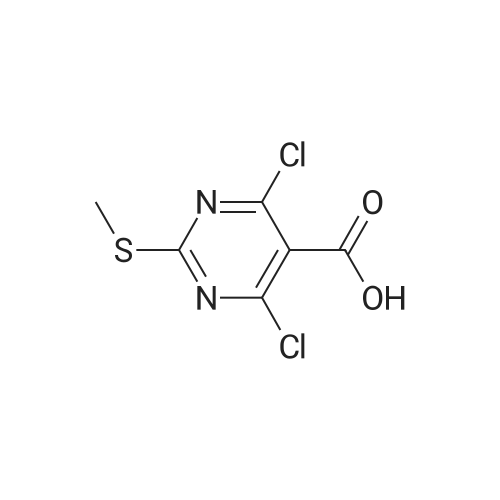
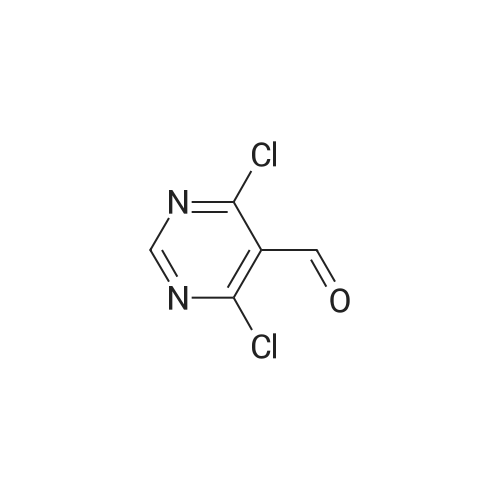
Step 2. Preparation of4-Chloro-6-methylsulfanyl-lH-pyrazolo[3,4-d]pyrimidine; 4,6-Dichloro-2-methylsulfanyl-pyrimidine-5-carbaldehyde (7.54 g, 0.0338 mol) was added to 80 mLof dioxane and stirred for 10 min at RT. Diisopropyl ethylamine (6.03 mL, 0.0340 mol) was added and the mixture was cooled in an ice bath with stirring for 10 min. Anhydrous hydrazine (1.08 mL, 0.0338 mmol) was added dropwise over three min, and stirring was continued for an additional five min. The ice bath was removed, and the reaction mixture was heated to reflux with stirring for 2 hours. The reaction mixture was then stirred at RT for 16 hours. The reaction mixture was concentrated under reduced pressure, and the residue was added to 20 mL of 2 N HCl and 100 mL EtOAc. The result- ing suspension was stirred and filtered, ad the solid was washed with water followed by EtOAc. The organic phase of the filtrate was collected, and the aqueous phase was extracted three times with 150 mL EtOAc. The combined organic phases were dried (MgSO4), filtered, and the filtrate was evaporated under reduced pressure. The resulting solid was washed with diethyl ether/hexanes (1:1) and the solid was dried to provide 3.13 g of crude 4-Chloro-6-methylsulfanyl-lH-pyrazolo[3,4-d]pyrimidine. Mass Spec. M-I-H = 201.Step 2. Preparation of4-Chloro-6-methylsulfanyl-lH-pyrazolo[3,4-d]pyrimidine; 4,6-Dichloro-2-methylsulfanyl-pyrimidine-5-carbaldehyde (7.54 g, 0.0338 mol) was added to 80 mL of dioxane and stirred for 10 min at RT. Diisopropyl ethylamine (6.03 mL,0.0340 mol) was added and the mixture was cooled in an ice bath with stirring for 10 min. Anhydrous hydrazine (1.08 mL, 0.0338 mmol) was added dropwise over three min, and stirring was continued for an additional five min. The ice bath was removed, and the reaction ixture was heated to reflux with stirring for two hours. The reaction mixture was then stirred at RT for 16 hours. The reaction mixture was concentrated under reduced pressure, and the residue was added to 20 mL of 2 N HCl and 100 mL EtOAc. The resulting suspension was stirred and filtered, ad the solid was washed with water followed by EtOAc. The organic phase of the filtrate was collected, and the aqueous phase was extracted three times with 150 mL EtOAc. The combined organic phases were dried (MgSO4), filtered, and the filtrate was evaporated under reduced pressure. The resulting solid was washed with diethyl ether/hexanes (1:1) and the solid was dried to provide 3.13 g of crude 4-Chloro-6-methylsulfanyl-lH-pyrazolo[3,4-d]pyrimidine. Mass Spec. M+H = 201. |
|
|
Step 2. Preparation of 4-Chloro-6-methylsulfanyl-]H-pyrazolo[3,4-d]pyrimidine 4,6-Dichloro-2-methylsulfanyl-pyrimidine-5-carbaldehyde (7.54 g, 0.0338 mol) was added to 80 mL of dioxane and stirred for 10 minutes at room temperature. Diisopropyl ethylamine (6.03 mL, 0.0340 mol) was added and the mixture was cooled in an ice bath with stirring for 10 minutes. Anhydrous hydrazine (1.08 mL, 0.0338 mmol) was added dropwise over three minutes, and stirring was continued for an additional five minutes. The ice bath was removed, and the reaction ixture was heated to reflux with stirring for two hours. The reaction mixture was then stirred at room temperature for 16 hours. The reaction mixture was concentrated under reduced pressure, and the residue was added to 20 mL of 2 N HCl and 100 mL EtOAc. The resulting suspension was stirred and filtered, ad the solid was washed with water followed by EtOAc. The organic phase of the filtrate was collected, and the aqueous phase was extracted three times with 150 mL EtOAc. The combined organic phases were dried (MgSO4), filtered, and the filtrate was evaporated under reduced pressure. The resulting solid was washed with diethyl ether/hexanes (1:1) and the solid was dried to provide 3.13 g of crude 4-Chloro-6-methylsulfanyl-1H-pyrazolo[3,4-d]pyrimidine. Mass Spec. M+H =201. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping