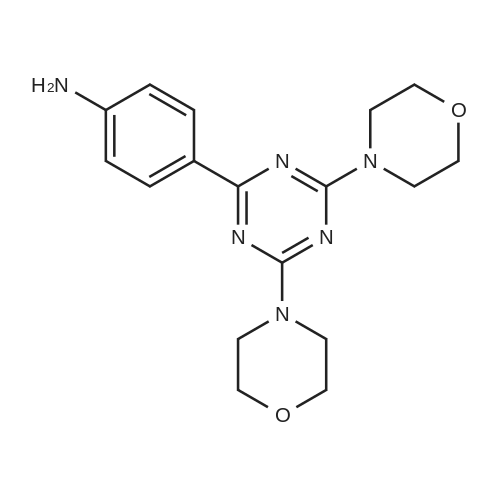
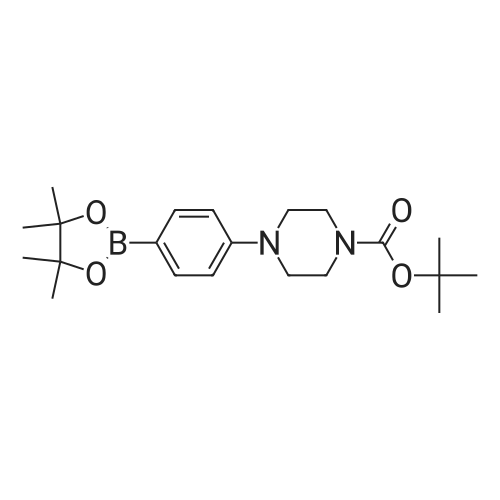
| 36% |
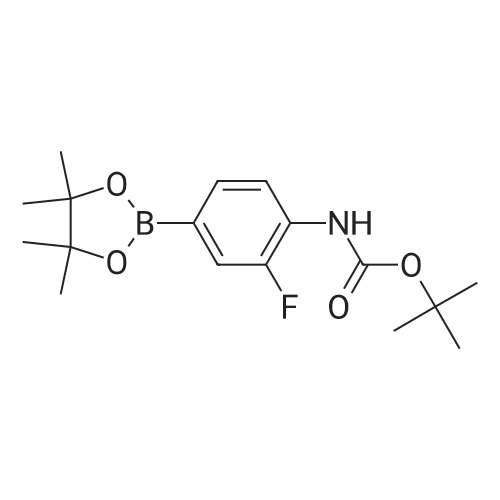
With (1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride; potassium acetate; In 1,4-dioxane; at 110℃; for 12h;Inert atmosphere; |
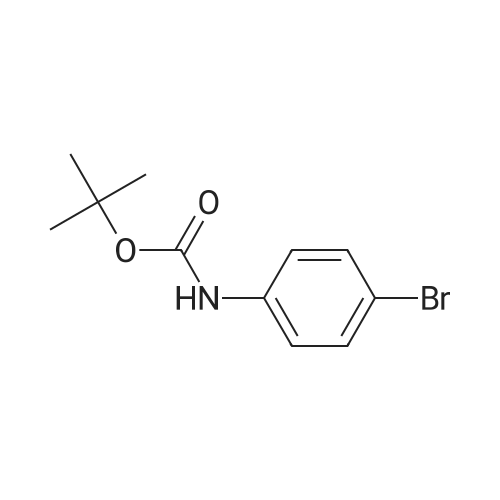
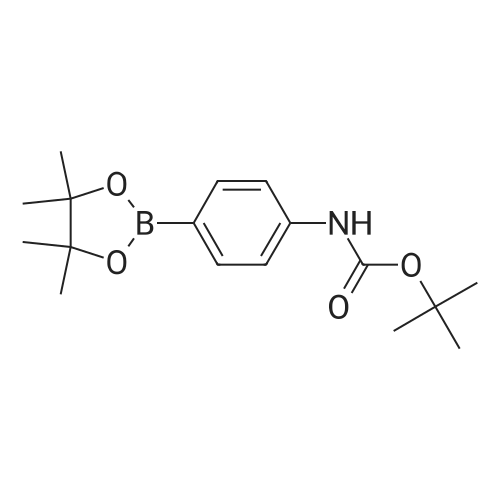
A mixture of bis(pinacolato)diboron (10.5 g, 41.5 mmol), 4 (7.75 g, 28.5 mmol), Pd(dppf)Cl2 (0.79 g, 1.1 mmol) and potassium acetate (7.0 g, 71.4 mmol) in dry dioxane (100 mL) was added into a 250 mL round bottom flask. The mixture was stirred for 12 h at 110 C under the protection of argon. After being cooled to room temperature, it was filtered and the filtrate was concentrated on a rotary evaporator. The residue was subjected to column chromatography over silica gel (PE/EA 10:1) to give 5 (3.26 g, 36%) as a white solid. 1H NMR (400 MHz, DMSO-d6) delta 9.53(s, 1H), 7.56(d, J = 8.5 Hz, 2H), 7.47(d, J = 8.5 Hz, 2H), 1.48(s, 9H), 1.29(s, 12H). |
|
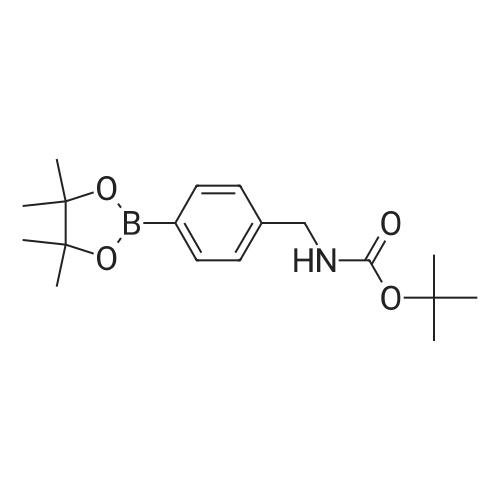
With potassium acetate; In n-heptane; dichloromethane; ethyl acetate; N,N-dimethyl-formamide; |
b tert-butyl N-[4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)phenyl]carbamate A mixture of tert-butyl N-[(4-bromophenyl)carbamate (5.95 g, 0.0219 mol), diboron pinacol ester (6.67 g, 0.0263 mol), [1.1'-bis(diphenylphosphino)ferrocene]-dichloropalladium (II) complex with dichloromethane (1:1) (0.536 g, 0.00066 mol) and potassium acetate (6.47 g, 0.066 mol) in N,N-dimethylformamide (120 mL) was heated at 80 C. under an atmosphere of nitrogen for 16 hours. The mixture was allowed to cool to ambient temperature and the solvent removed under reduced pressure. Dichloromethane (100 mL) was added to the residue and the resulting solid was removed by filtration through a pad of Celite. The filtrate was concentrated to leave a yellow oil which was purified by flash chromatography on silica using ethyl acetate/n-heptane (7:93) as mobile phase. The resulting fractions were concentrated, the residue was triturated in n-heptane and the precipitate collected by filtration to yield tert-butyl N-[4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)phenyl]carbamate (6.0 g, 0.0188 mol) as a white solid. 1H NMR (DMSO-d6, 400 MHz) delta 9.50(s, 1H), 7.55 (d, 2H), 7.46 (d, 2H), 1.47 (s, 9H), 1.27 (s, 12H). |
|
With potassium acetate; In n-heptane; dichloromethane; ethyl acetate; N,N-dimethyl-formamide; |
b) tert-butyl N-[4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)phenyl]carbamate A mixture of tert-butyl N-[(4-bromophenyl)carbamate (5.95 g, 0.0219 mol), diboron pinacol ester (6.67 g, 0.0263 mol), [1.1'-bis(diphenylphosphino)ferrocene]-dichloropalladium (II) complex with dichloromethane (1:1) (0.536 g, 0.00066 mol) and potassium acetate (6.47 g, 0.066 mol) in N,N-dimethylformamide (120 mL) was heated at 80 C. under an atmosphere of nitrogen for 16 hours. The mixture was allowed to cool to ambient temperature and the solvent removed under reduced pressure. Dichloromethane (100 mL) was added to the residue and the resulting solid was removed by filtration through a pad of Celite. The filtrate was concentrated to leave a yellow oil which was purified by flash chromatography on silica using ethyl acetate/n-heptane (7:93) as mobile phase. The resulting fractions were concentrated, the residue was triturated in n-heptane and the precipitate collected by filtration to yield tert-butyl N-[4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)phenyl]carbamate (6.0 g, 0.0188 mol) as a white solid. 1H NMR (DMSO-d6, 400 MHz) delta 9.50(s, 1H), 7.55 (d, 2H), 7.46 (d, 2H), 1.47 (s, 9H), 1.27 (s, 12H). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping