| 85% |
In acetone; at 40℃; for 5h; |
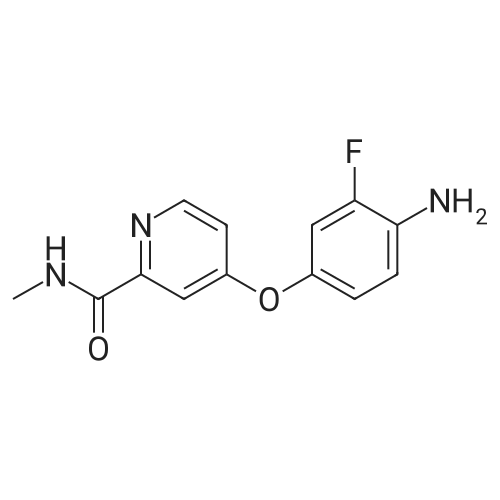
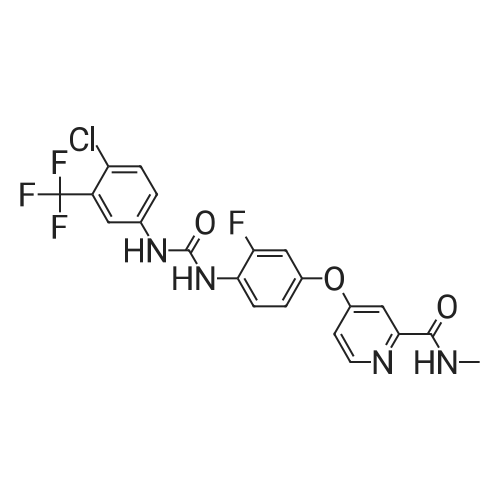
EXAMPLE 7 (0037) Under the protection of nitrogen, 171.5 g of 4-(4-amino-3-fluorophenoxy)-N-methylpyridine-2-formamide and 1200 ml of acetone are added to a reaction vessel equipped with a stirrer, a thermometer and a dropping funnel, which is kept at 40 C., and then slowly add dropwise 159.5 g of 4-chloro-3-(trifluoromethyl) phenyl isocyanate in 600 ml of acetone solution (completed in about 2 hours), and then maintain the temperature and stir again for another 3 hours, filter to obtain a filter cake, which is then dried in vacuum to obtain 269 g of a white or off-white solid of Regorafenib. HPLC content is 99.4%, mp: 187 to 188 C., yield 85%. |
| 74.9% |
In tetrahydrofuran; toluene; at 27℃; for 4.5h; |
4-(4-amino-3-fluorophenoxy)-N-methylpicolinamide (30 g) was dissolved in tetrahydrofuran (150 mL) at 27C and the solution containing 4-chloro-3 (trifluoromethyl) phenyl isocyanate (28 g) in toluene (36 mL) was added slowly at 27C in 90 minutes. The reaction mass was stirred for 3 hours at same temperature. After completion of reaction, tetrahydrofuran (51 mL) and methanol (15 mL) were added to the reaction mixture at 27C. Acetyl chloride (12 mL) was added drop wise to the reaction mixture at 27C for 10 minutes and stirred for 1 hour at same temperature. Compound was filtered and washed with tetrahydrofuran (30 mL) and then with acetone (140 mL). The obtained compound was dissolved in mixture of tetrahydrofuran (280 mL), 45% w/w aqueous sodium hydroxide solution (12 g) and water (80 mL) at 40C and stirred the reaction mass for 30 mm at 40C. The reaction mass was cooled to 20C and water (50 mL) was added. The reaction mass was further cooled to 0C and stirred at same temperature for 15 minutes. Reaction mass was distilled to completely evaporate the solvent at 35C and then the reaction mass was co-distilled with acetone (30 mL) at 35C. Acetone (280 mL) was added to the reaction mass at 27C and stirred for 15 minutes at same temperature. Reaction mass was cooled to 0C and stirred for 30 mm at same temperature. The solid was filtered and washed with chilled mixture of water (65 mL) and acetone (160 mL). The wet compound was dried for 2 hours at 25-30C to obtain the title compound. Yield: 41 .5 g (74.9 %); HPLC purity: 99.22% |
| 73.46% |
In tetrahydrofuran; at 30℃; for 12h; |
4-Chloro-3-(trifluoromethyl)phenylisocyanate (101.8 g) was dissolved in THF (200 ml) andadded to the solution of 4-(4-amino-3-fluorophenoxy)-N-methylpyridine-2-carboxamide in THF (800 ml) at 30C and stirred for 12h.The reaction was monitored by LC-MS analysis and controlled 4-(4-amino-3 -fluorophenoxy)-N-methylpyridine-2-carboxamide content to NMT 50 ppm during reaction. The reaction mass was concentrated and co-distilled with acetone (2 x 200 ml). The concentrated mass was dissolved in acetone (4000 ml) at 55C,treated with activated carbon (15 g) for 30 mm and filtered through hyflo bed. The filtrate was partially concentrated, cooled to 20C, filtered and dried the product to yield crude Regorafenib anhydrous form 1(135.9 g, 73.46%). HPLC purity: 99.43 1% |
| 47% |
In toluene; at 20℃; for 72h; |
To a solution of 4- (4-AMINO-3-FLUOROPHENOXY) pyridine-2-carboxylic acid methylamide (177 mg, 0.68 mmol) in toluene (3 mL) was added 4-chloro-3-(trifluoromethyl) phenyl isocyanate (150. MG, 0.68 mmol). The mixture was stirred at rt for 72 h. The reaction was concentrated under reduced pressure and the residue was triturated with diethylether. The resulting solid was collected by filtration and dried in vacuo for 4 h to afford the title compound (155 MG,. 0. 32 mmol; 47% yield) ; H-NMR (DMSO-d6) 2. 78 (d, J=4.9, 3H), 7.03-7. 08 (M, 1H), 7.16 (dd, J=2.6, 5.6, 1H), 7.32 (dd, J=2.7, 11.6, 1H), 7.39 (d, J=2.5, 1H), 7.60 (s, 2H), 8.07-8. 18 (M, 2H), 8. 50 (d, J=5. 7,1H), 8.72 (s, 1H), 8.74-8. 80 (M, 1H), 9.50 (s, 1H) ; MS (HPLC/ES) 483.06 m/z = (M + 1) |
| 47% |
In toluene; at 20℃; for 72h; |
To a solution of 4-(4-amino-3-fluorophenoxy)pyridine-2-carboxylic acid methylamide (starting material 1, 177 mg, 0.68 mmol) in toluene (3 mL) was added 4-chloro-3-(trifluoromethyl)phenyl isocyanate (150 mg, 0.68 mmol). The mixture was stirred at room temperature for 72 h. The reaction was concentrated under reduced pressure and the residue was triturated with diethylether. The resulting solid was collected by filtration and dried in vacuo for 4 h to afford the title compound (155 mg, 0.32 mmol; 47% yield); 1H-NMR (DMSO-d6) 2.78 (d, J=4.9, 3H), 7.03-7.08 (m, 1H), 7.16 (dd, J=2.6, 5.6, 1H), 7.32 (dd, J=2.7, 11.6, 1H), 7.39 (d, J=2.5, 1H), 7.60 (s, 2H), 8.07-8.18 (m, 2H), 8.50 (d, J=5.7, 1H), 8.72 (s, 1H), 8.74-8.80 (m, 1H), 9.50 (s, 1H); MS (HPLC/ES) 483.06 m/z = (M + 1). |
| 47% |
In toluene; at 20℃; for 72h; |
To a solution of 4-(4-amino-3-fluorophenoxy)pyridine-2-carboxylic acid methylamide (starting material 1 , 177 mg, 0.68 mmol) in toluene (3 ml_) was added 4-chloro-3- (trifluoromethyl)phenyl isocyanate (150 mg, 0.68 mmol). The mixture was stirred at room temperature for 72 h. The reaction was concentrated under reduced pressure and the residue was triturated with diethylether. The resulting solid was collected by filtration and dried in vacuo for 4 h to afford the title compound (155 mg, 0.32 mmol; 47% yield); 1H- NMR (DMSO-de) 2.78 (d, J=4.9, 3H), 7.03-7.08 (m, 1 H), 7.16 (dd, J=2.6, 5.6, 1 H), 7.32 (dd, J=2.7, 11.6, 1 H), 7.39 (d, J=2.5, 1 H), 7.60 (s, 2H), 8.07-8.18 (m, 2H), 8.50 (d, <n="16"/>J=5.7, 1 H), 8.72 (s, 1 H), 8.74-8.80 (m, 1 H), 9.50 (s, 1 H); MS (HPLC/ES) 483.06 m/z = (M + 1 ). |
| 47% |
In toluene; at 20℃; for 72h; |
To a solution of 4-(4-amino-3-fluorophenoxy)pyridine-2-carboxylic acid methylamide (177 mg, 0.68 mmol) in toluene (3 mL) was added 4-chloro-3-(trifluoromethyl)phenyl isocyanate (150 mg, 0.68 mmol). The mixture was stirred at room temperature for 72 h. The reaction was concentrated under reduced pressure and the residue was triturated with diethylether. The resulting solid was collected by filtration and dried in vacuo for 4 h to afford the title compound (155 mg, 0.32 mmol; 47% yield); 1H-NMR (DMSO-d6) 2.78 (d, J=4.9, 3H), 7.03-7.08 (m, 1H), 7.16 (dd, J=2.6, 5.6, 1H), 7.32 (dd, J=2.7, 11.6, 1H), 7.39 (d, J=2.5, 1H), 7.60 (s, 2H), 8.07-8.18 (m, 2H), 8.50 (d, J=5.7, 1H), 8.72 (s, 1H), 8.74-8.80 (m, 1H), 9.50 (s, 1H); MS (HPLC/ES) 483.06 m/z=(M+1). |
| 47% |
In toluene; at 20℃; for 72h; |
Example 1: Preparation of 4{4-[3-(4-chloro-3-trifluoromethylphenyl)-ureido]-3- fluorophenoxy}-pyridine-2-carboxylic acid methylamide; To a solution of 4-(4-amino-3-fluorophenoxy)pyridine-2-carboxylic acid methylamide (177 mg, 0.68 mmol) in toluene (3 mL) was added 4-chloro-3-(trifluoromethyl)phenyl isocyanate (150 mg, 0.68 mmol). The mixture was stirred at rt for 72 h. The reaction was concentrated under reduced pressure and the residue was triturated with diethylether. The resulting solid was collected by filtration and dried in vacuo for 4 h to afford the title compound (155 mg, 0.32 mmol; 47% yield); 1H-NMR (DMSO-d6) 2.78 (d, J=4.9, 3H), 7.03-7.08 (m, 1H), 7.16 (dd, J=2.6, 5.6, 1H), 7.32 (dd, J=2.7, 11.6, 1H), 7.39 (d, J=2.5, 1H), 7.60 (s, 2H), 8.07-8.18 (m, 2H), 8.50 (d, J=5.7, 1H), 8.72 (s, 1H), 8.74-8.80 (m, 1H), 9.50 (s, 1H); MS (HPLC/ES) 483.06 m/z = (M + 1). |
|
In ethyl acetate; at 20 - 45℃; for 1.5h; |
Example 1: Preparation of 4- [4-([4-chloro-3-(trifluoromethyl)phenyll carbamoyl} amino)-3- fluorophenoxyl-N-methylpyridine-2-carboxamide in the polymorph IIExample 11O g 4-(4-amino-3-fluorophenoxy)pyridine-2-carboxylic acid methylamide, obtained as described in WO 2005/009961 , are suspended in ethyl acetate at room temperature. The suspension is heated to 400C and 8,84 g of the commercially available 4-Chlor-3-(trifluormethyl)-phenylisocyanate solved in 10 g ethyl acetate are added within 45 min. at 40 - 45C. The suspension is cooled within 45 min. to room temperature. The crystals are sucked off, washed with ethyl acetate and dried 15 h at room temperature and 40 mbar. The product is tested thermoanalytically (DSC) and corresponds to the title compound of formula (I) in the polymorph ?. |
| 350 g |
In dichloromethane; at 0 - 5℃; for 4h;Inert atmosphere; |
4-chloro-3-(trifloromethyl)phenyl isocyanate (318.0) and 4-(4-amino-3-fluorophenoxy)-N-methylpicolinamide (125 g) were added to a pre-cooled dichioromethane (2500 ml) at 0C to 5C under nitrogen atmosphere. Stirred the reaction mixture for 60 minutes at the same temperature. Another lot of 4-(4-amino-3-fluorophenoxy)-N-methylpicolinamide (125 g) was added to the reaction mixture at 0C to 5C and stirred the reaction mixture for 3 hours. Filtered the solid and washed with dichloromethane and dried the compound at 35C to 40C for 4 hours to get the title compound. Yield: 350 g;Content of 4-(4-amino-3 -fluorophenoxy)-N-methylpicolinamide: 704.13 ppm (0.070 % by HPLC) |
| 5.5 g |
In acetone; at 25 - 30℃; |
4-(4-amino-3-fluorophenoxy) pyridine-2-carboxylic acid methyl amide (4g, 0.01 moles) was added in to a reaction flask containing acetone (20 ml) at 25-30C and stirred for 15 minutes. 4-chloro-3-trifluoromethylisocyanate (6.1g, 0.02 moles) was added slowly over a period of 5 to 10 minutes and stirred the reaction mixture 3 to 4 hours. Toluene (20 n L) was added to the reaction mass and stirred for 30 min at 25-30C.The obtained reaction mass was filtered and washed with toluene (8 mL). Dried the material still constant weight appears to yield title product a crystalline material. (0106) Yield: 5.5 gm (0107) Chromatographic Purity (By HPLC): 97% |
|
In ethyl acetate; at 20℃; for 0.5h; |
the 3.0g (11mmol) of Intermediate 7 was dissolved in 20mL ethyl acetate was added 3.5g (16mmol)) 4- chloro-3-trifluoromethylphenyl isocyanate (8), at room temperature the reaction 30min, suction filtered, washed with 50mL diethyl ether cake filtration, dried to give a pale pink powder (1).Yield 94.5%, 99.8% purity |
|
|
Join with stirringRegiofenib intermediate 1 and its weight 8.300 times the amount of tetrahydrofuran in the reaction vessel,Replace the nitrogen,Control temperature is 25 C,Intravenous addition of rigofene intermediate 1 within 25 min, 32% by weight of 2.948 times4-chloro-3-(trifluoromethyl)benzene isocyanate- tetrahydrofuran solution, reaction at 25 C for 3 h,Adding rigofene intermediate 1 weight 0.906 times the amount of methanol, reacting at 25 C for 1 h;Adding ryogofibine intermediate 1 by weight of 0.457 times of hydrochloric acid,Reacted at 25 C for 4 h,Filter by suction, wash, and dry. |
|
|
Under the protection of nitrogen,RGFN 1 and dichloromethane were added to the reaction vessel, and the molar ratio of RGFN 1: dichloromethane was 1:12-18.Stir in the reactor at a temperature of 25 to 30 C for 15 to 20 minutes.Then open the circulating refrigeration oil bath valve of the reactor jacket or circulating chilled water (?-10 C) to cool the system temperature to 0 C.Further adding 4-chloro-3-(trifluoromethyl)benzene isocyanate (RGFN-C for short),The molar ratio of RGFN 1:RGFN-C is 1:1 to 1.2.Stir the reaction at 0 C or below for 15-20 min,The reaction kettle was closed and stirred for 30 min.Close the circulating refrigeration oil bath valve or turn off the circulating oil bath refrigeration to open the circulating oil bath heating switch or open the reactor jacket circulating cooling water, slowly heat up, control the temperature in the reactor system 25 ~ 30 C, continue to stir the reaction for 2h,Then open the reaction vessel jacketed circulating refrigeration oil bath valve or cycle refrigerationWater (?-10 C) is cooled to the temperature of the reactor. The temperature of the system is below 0 C. The mixture is stirred and decanted for 12 hours. The reactor is closed and stirred. The bottom of the reactor is opened, and the solid-liquid mixture in the reactor is centrifuged or filtered. The filter cake is washed 2 to 4 times with dichloromethane previously pre-frozen to a temperature of 0 C or below.Each time the amount of dichloromethane is 1 times the weight of RGFN 2, the cake is turnedMove to the drying tray in the vacuum drying box, crush the larger block, spread evenly, spread the thickness ? 30mm, 0.075MPa ~ 0.1MPa vacuum under vacuum and dry, the drying temperature is 25 ~ 30 C Dry under reduced pressure for 2h~4h,4-[4-([4-chloro-3-(trifluoromethyl)phenyl)carbamoyl}amino)-3-fluorophenylamino]-N-methylpyridine-2-carboxamide is obtained,That is, intermediate 2 (abbreviated as RGFN 2), the yield is 80-90%. |
| 86.7 g |
In tetrahydrofuran; at 20℃; for 5h; |
In a 500 mL closed four-neck reaction flask, 31.4 g of 3-fluoro-4-nitrophenol (II), 34.1 g of N-methyl-4-chloro-2-pyridinecarboxamide, 27.8 g of potassium carbonate and 150 g were added. Diethylene glycol dimethyl ether, heating under reflux, stirring reaction for 6 hours, adding 200 g of water to precipitate a solid at the end of the reaction, and filtering to obtain 4-(4-nitro-3-fluorophenoxy)-2-(methylamine) 56.4 g of crude formyl)pyridine (III),Add 56.4 grams of III obtainedIn a 500 mL closed high pressure autoclave, 1.16 g of Raney nickel and 200 g of methanol were passed through 2 atm of hydrogen, and the temperature was controlled at 20 C. The reaction was stirred for 3 hours. The reaction was filtered to recover Raney nickel, and the methanol was distilled off under reduced pressure to obtain 50.2 g. IV,Then 50.2 g of IV was added to a 500 mL closed four-neck reaction flask, and 42.6 g of 4-chloro-3-(trifluoromethyl)phenylisocyanate and 200 g of tetrahydrofuran were added.The reaction was stirred at room temperature for 20 hours at 20 C, and 75 g of 10% dilute hydrochloric acid was added dropwise to the end of the reaction to precipitate a solid. The residue was filtered to obtain the hydrochloride salt of the product, the sodium carbonate aqueous solution was free and then extracted with ethyl acetate. The ester product was 83.7 g of regomafenib with a total yield of 86.4%. |
| 31.5 g |
|
To a reaction flask with a stirrer was added 20.0 g of 4- (4-amino-3-fluorophenoxy) -N-methylpyridine-2-carboxamide prepared by stage 2 method 2a (i.e. formula (IV) Compound), glacial acetic acid (molar ratio of the compound of formula (IV) to glacial acetic acid is 1: 0.15) and 180 g of tetrahydrofuran as a solvent. A solution of 18.7 g of (4-chloro-3-trifluoromethyl-phenyl) isocyanate (compound of formula (V)) and 21.1 g of toluene was added dropwise at room temperature over approximately 90 minutes. The resulting solution was stirred for 3 hours to complete the reaction.30 g of tetrahydrofuran and 7.8 g of methanol were then added to the reaction mixture. Then 9.0 g of acetyl chloride was added dropwise to the reaction mixture over 15 minutes. After stirring for an additional 2 hours, the suspension was filtered and the solid was washed with tetrahydrofuran (18.2 g) and acetone (136.4 g).The solid was added to a mixture of acetone (268.6 g), water (55.8 g) and aqueous sodium hydroxide solution (8.2 g, 45% w / w) at 40 C. The mixture was stirred for an additional 30 minutes. Then by using 4- {4-[([4-chloro-3- (trifluoromethyl) -phenyl] amino} carbonyl) amino] -3-fluorophenoxy} -N-methylpyridine-2 -Seeding of crystals of carboxamide monohydrate initiates crystallization. After cooling to 20 C, 31.6 g of water were added. The suspension was cooled to about 3 C and stirred for 30 minutes.The product was filtered off, washed with a cold mixture of acetone (106 g) and water (44 g) and dried under reduced pressure (30 C, 80 mbar).In this way, 31.3 g of the product 4- {4-[([4-chloro-3- (trifluoromethyl) -phenyl] amino} carbonyl) amino] -3-fluorophenoxy} -N -Methylpyridine-2-carboxamide monohydrate as white crystals. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science













 HazMat Fee +
HazMat Fee +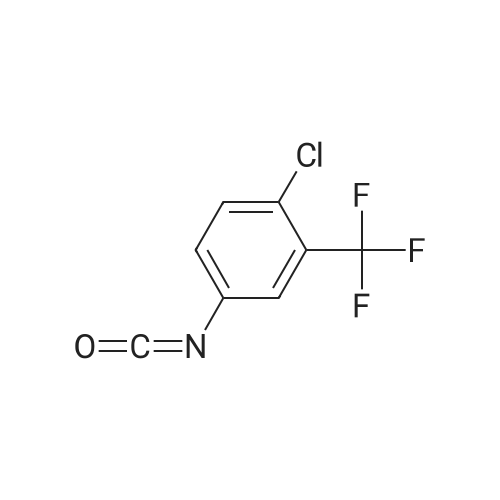

 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping