| 95% |
With tri-n-propylamine; trichlorophosphate; In toluene; at 20 - 110℃; |
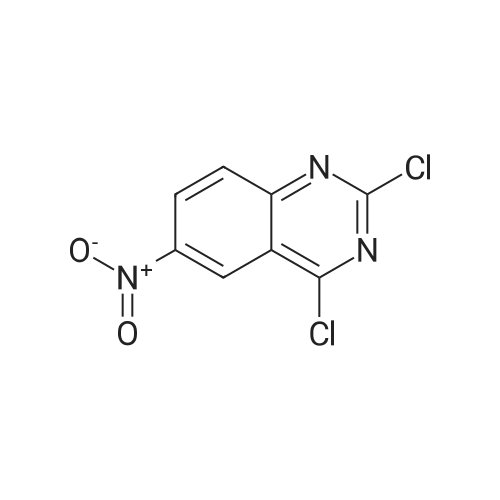
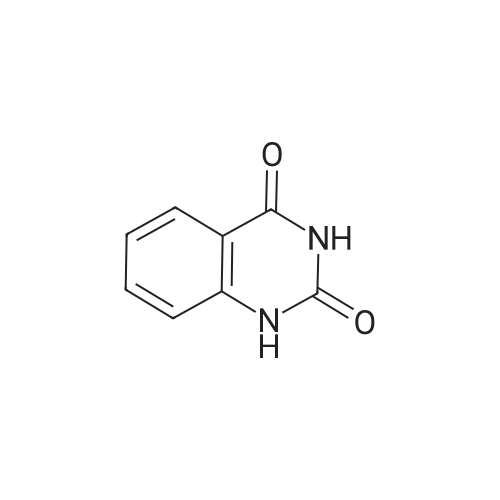
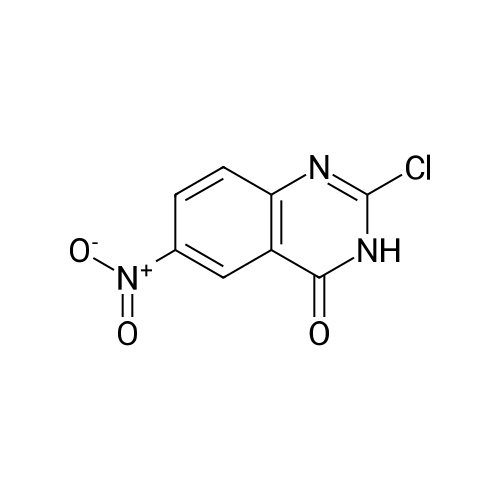
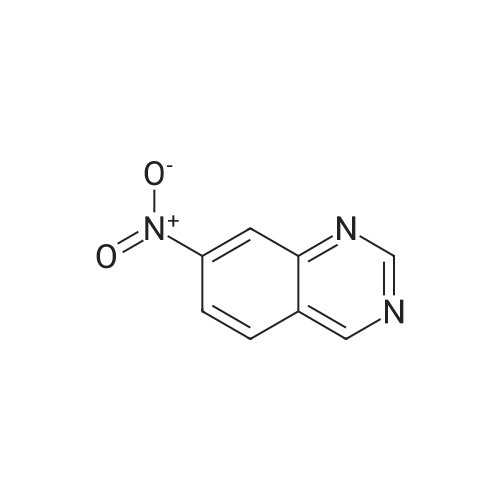
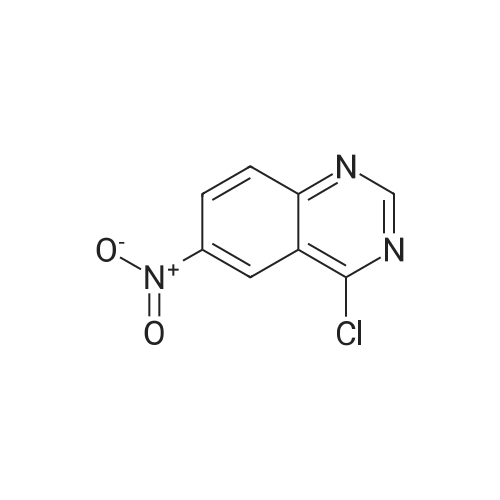
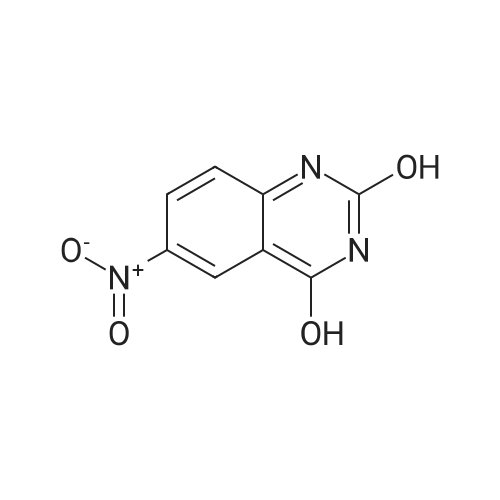
Step B: Preparation of 2,4-dichloro-6-nitro-quinazoline. Phosphorus oxychloride (6.64 mL, 72.6 mmol) was added to a suspension of 6-nitro-1 H-quinazoline-2,4-dione (5.01 g, 24.2 mmol) in toluene (100 mL) and the reaction mixture was heated to 55 C. Tri-n-propylamine (12.1 mL, 63.9 mmol) was added dropwise from an addition funnel over 25 minutes. The reaction mixture was heated to 110 C. for 6 h, stirred at room temperature for 4 d, and then pipetted into water (75 mL) and vigorously stirred for 1 h. The two layers were filtered and separated. The organic layer was washed with brine (30 mL), dried (MgSO4), and concentrated to yield the titled compound (3.79 g, 67% yield, 95% pure) after 24 h under high vacuum. This compound did not yield MS data. 1H NMR (600 MHz, CDCl3): 9.18 (d, J=2.4 Hz, 1H), 8.75 (dd, J=9.2, 2.5 Hz, 1H), 8.18 (d, J=9.2 Hz, 1H). |
| 63% |
With phosphorus pentachloride; trichlorophosphate; for 6.5h;Reflux; |
The reaction mixture of compound 2a (0.78 g, 3.78 mmol), PCl5 (4.11 g, 19.7 mmol) and POCl3 (16 mL) was stirred at reflux for 6.5 h. The excess POCl3 was removed by evaporation. The residue was dissolved in ice water, and then the solution pH was adjusted to pH 5-6 with saturated NaHCO3. The water phase was extracted with EtOAc (60 mL × 5) and the organic layer was dried over anhydrous Na2SO4, concentrated to give the crude product which was purified by column chromatography on silica gel (petroleum ether/EtOAc = 40:1) to afford compound 3a as white solid (0.58 g, 63%); mp 122-124 C; 1H NMR (CDCl3) delta: 8.18 (d, J = 9.0 Hz, 1H), 8.76 (dd, J1 = 9.3 Hz, J2 = 2.1 Hz, 1H), 9.18 (d, J = 1.8 Hz, 1H). |
| 63.5% |
|
General procedure: 6-Nitroquinazoline-2,4 (1H, 3H) -dione (0.782 g, 3.78 mmol),Phosphorus pentachloride (4.111 g, 19.74 mmol),Add to 16 mL of phosphorus oxychloride,The reaction mixture was heated to reflux for 6.5 h and worked up as in Example 1 (b).0.585 g of a white solid was obtained in a yield of 63.5%. 1H-Quinazolin-2,4-one (0.5 g, 3.09 mmol) and phosphorus oxychloride (4.3 mL) were added to the reaction flask. After stirring for 0.5 h, 1.6 mL of N, N-dimethylaniline was added and the mixture was refluxed Reaction about 7h. The excess phosphorus oxychloride was distilled off under reduced pressure, and the remaining phosphorus oxychloride was taken out with chloroform. The residue was dissolved in ethyl acetate, and the excess N, N-dimethylaniline was removed with cold dilute hydrochloric acid to separate the water Phase, the organic phase was adjusted with saturated sodium bicarbonate pH = 5-6, the aqueous phase was extracted with ethyl acetate, the organic phase was combined, washed sequentially with saturated aqueous sodium chloride solution, the organic phase was dried over anhydrous magnesium sulfate, Mobile phase: ethyl acetate / petroleum ether = 50/1) to give crude product 0.649g. The crude product was recrystallized from 6 mL of methanol to give 0.489 g of a yellow flocculent solid. Yield: 79.6%. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping