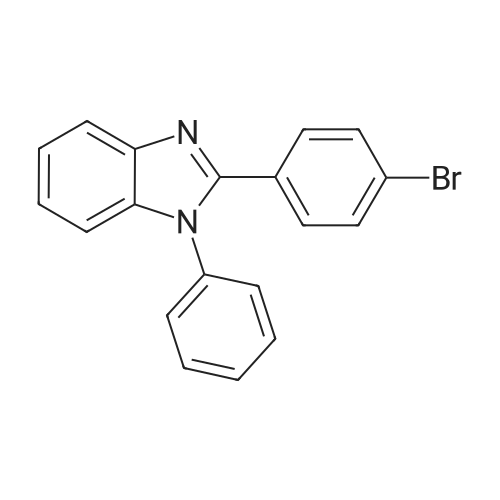
| 82% |
With tri-tert-butyl phosphine; sodium t-butanolate;bis(dibenzylideneacetone)-palladium(0); In hexane; xylene; at 130℃; for 5h;Inert atmosphere; |
In a 100 mL three-neck flask were put 1.7 g (5.0 mmol) of <strong>[2620-76-0]2-(4-bromophenyl)-1-phenyl-1H-benzimidazole</strong>, 1.2 g (5.0 mmol) of phenyl biphenylamine, 1.0 g (10 mmol) of sodium tert-butoxide, and 17 mg (30 μmol) of bis(dibenzylideneacetone)palladium(0), and the air in the flask was replaced with nitrogen. Then, 10 mL of dehydrated xylene was added to this mixture. After the mixture was deaerated while being stirred under reduced pressure, 200 mL (0.1 mmol) of tri(tert-butyl)phosphine (10 wt % hexane solution) was added thereto. This mixture was stirred under a nitrogen atmosphere at 130 C. for 5 hours to be reacted.After the reaction, 200 mL of ethyl acetate was added to this reaction mixture, and this suspension was filtered through Florisil and Celite. The obtained filtrate was concentrated and purified by silica gel column chromatography (developing solvent, toluene:ethyl acetate=9:1). The obtained fraction was concentrated, and acetone and methanol were added thereto. The mixture was irradiated with supersonic and then recrystallized, so that the object of the synthesis was obtained as 2.1 g of a light-yellow powder in a yield of 82%.A reaction scheme of the above synthesis method is illustrated in the following scheme (A-1). Results of nuclear magnetic resonance spectrometry (1H-NMR), by which the compound obtained by the above synthesis method was analyzed, are shown below. In addition, the 1H-NMR charts are shown in FIGS. 8A and 8B. FIG. 8B illustrates an enlarged view within a range of 6 ppm to 9 ppm in FIG. 8A. The results reveal that 4-phenyl-4'-(1-phenyl-1H-benzimidazol-2-yl)-phenyl-triphenylamine (abbreviation: BPABIm), which is the triarylamine compound of one embodiment of the present invention represented by the structural formula (100) shown above, was obtained.1H NMR (CDCl3, 300 MHz): δ (ppm)=6.98 (d, J=9.0 Hz, 2H), 7.07 (t, J=7.2 Hz, 1H), 7.12-7.58 (m, 25H), 7.85 (d, J=7.8 Hz, 1H).Next, ultraviolet-visible absorption spectra (hereinafter, simply referred to as “absorption spectra”) and emission spectra of BPABIm were measured. The absorption spectra were measured using an ultraviolet-visible light spectrophotometer (V550 type manufactured by Japan Spectroscopy Corporation). The emission spectra were measured using a fluorescence spectrophotometer (FS920 manufactured by Hamamatsu Photonics Corporation). The absorption spectra and the emission spectra of a toluene solution of BPABIm and a thin film of BPABIm were measured. Put in a quartz cell, the toluene solution (0.120 mmol/L) was subjected to the measurement at room temperature. As for the measurement of the absorption spectrum of the thin film, the thin film which was evaporated over a quartz substrate was used and a value obtained by subtraction of an absorption spectrum of quartz from absorption spectra of the thin film and quartz is shown.FIGS. 9A and 9B show measurement results of the absorption spectra and emission spectra. FIG. 9A shows the measurement results of the toluene solution of BPABIm. FIG. 9B shows the measurement results of the thin film of BPABIm. In each of FIGS. 9A and 9B, the horizontal axis represents wavelength (nm) and the vertical axis represents absorption intensity (arbitrary unit) or emission intensity (arbitrary unit). In each of FIGS. 9A and 9B, the two solid lines are shown, and the thin line represents absorption spectrum while the thick line represents emission spectrum.In the case of the toluene solution of BPABIm, an absorption peak is observed at around 361 nm as shown in FIG. 9A. In the case of the thin film of BPABIm, an absorption peak is observed at around 364 nm as shown in FIG. 9B.Further, in the case of the toluene solution of BPABIm, the maximum emission wavelength is 410 nm (excitation wavelength: 365 nm) as shown in FIG. 9A. In the case of the thin film of BPABIm, the maximum emission wavelength is 445 nm (excitation wavelength: 363 nm) as shown in FIG. 9B.As described above, BPABIm was found to emit blue light with high color purity and accordingly can be used for a blue light-emitting material.Further, the HOMO level and the LUMO level of BPABIm were obtained by cyclic voltammetry (CV) measurements. An electrochemical analyzer (ALS model 600A or 600C, manufactured by BAS Inc.) was used for the CV measurements.Further, as for a solution used for the CV measurements, dehydrated dimethylformamide (DMF, manufactured by Sigma-Aldrich Inc., 99.8%, Catalog No. 22705-6) was used as a solvent, and tetra-n-butylammonium perchlorate (n-Bu4NClO4, manufactured by Tokyo Chemical Industry Co., Ltd., Catalog No. T0836), which was a supporting electrolyte, was dissolved in the solvent such that the concentration of tetra-n-butylammonium perchlorate was 100 mmol/L. Further, the object to be measured was dissolved in the solvent such that the concentration thereof was 2 mmol/L. A platinum electrode (PTE platinum electrode, manufactured by BAS Inc.) was used as a working electrode, a platinum electrode (Pt counter ... |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping