| 86% |
With hydrogen;palladium 10% on activated carbon; In ethanol; under 2585.81 Torr; for 1h; |
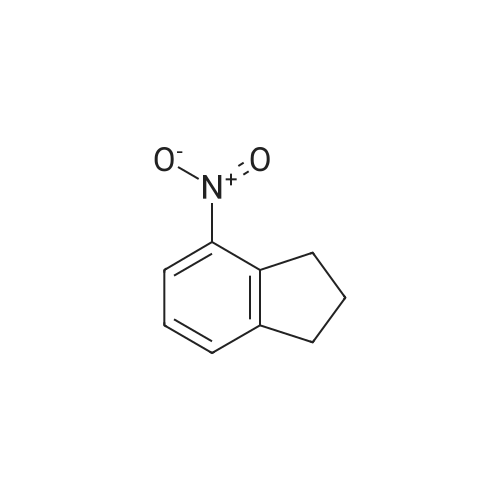
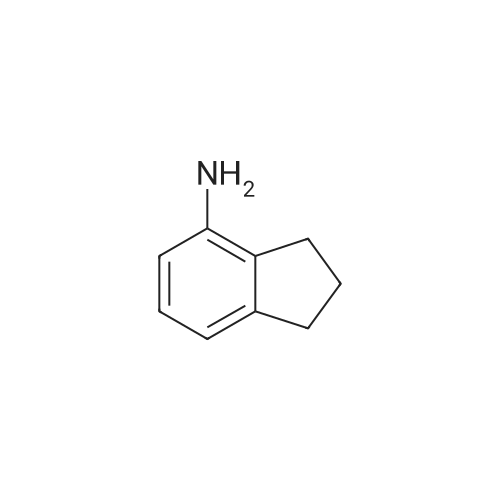
Intermediate 8: 4-aminoindane In a 500 mL Parr shaker vessel, 4-nitroindane (10 g, 61 mmol) was dissolved in 50 mL ethanol. A slurry of 10% Pd/C (1 g) in ethanol was added. The mixture was then placed on a Parr shaker under a hydrogen atmosphere (50 psi) for 1 hour, at which point t.l.c. (20% ethyl acetate in hexanes) showed that all the starting material had disappeared. To work up the reaction, the mixture was filtered twice through Celite, washing with a large amount of ethanol, and once through filter paper. The ethanol was evaporated under reduced pressure, and the crude product purified by flash chromatography over silica gel (10% ethyl acetate in hexanes) to give 8 as a viscous, faintly colored oil (7.04 g, 86% yield): 1H NMR (400 MHz, DMSO-D6) delta 1.95 (m, 2H) 2.61 (t, J=7.3 Hz, 2H) 2.76 (t, J=7.5 Hz, 2H) 4.77 (s, 2H) 6.36 (d, J=7.8 Hz, 1H) 6.42 (d, J=6.8 Hz, 1H) 6.80 (t, J=7.6 Hz, 1H). |
| 86% |
With hydrogen;palladium 10% on activated carbon; In ethanol; under 2585.81 Torr; for 1h; |
In a 500 mL Parr shaker vessel, 4-nitroindane (10 g, 61 mmol) was dissolved in 50 mL ethanol. A slurry of 10% Pd/C (1 g) in ethanol was added. The mixture was then placed on a Parr shaker under a hydrogen atmosphere (50 psi) for 1 hour, at which point t.l.c. (20% ethyl acetate in hexanes) showed that all the starting material had disappeared. To work up the reaction, the mixture was filtered twice through Celite, washing with a large amount of ethanol, and once through filter paper. The ethanol was evaporated under reduced pressure, and the crude product purified by flash chromatography over silica gel (10% ethyl acetate in hexanes) to give 8 as a viscous, faintly colored oil (7.04 g, 86% yield): 1H NMR (400 MHz, DMSO-D6) delta 1.95 (m, 2H) 2.61 (t, J=7.3 Hz, 2H) 2.76 (t, J=7.5 Hz, 2H) 4.77 (s, 2H) 6.36 (d, J=7.8 Hz, 1H) 6.42 (d, J=6.8 Hz, 1H) 6.80 (t, J=7.6 Hz, 1H). |
| 43% |
With palladium 10% on activated carbon; hydrogen; In methanol; at 20℃; under 2585.81 Torr; for 12h;Inert atmosphere; |
To a solution of 4-nitro-2,3-dihydro-1H-indene g, 337.07 mmol, 1 eq) in MeOH(500 mL) was added Pd/C ( g, 10% purity) under N2. The suspension was degassed invacuo and purged with H2 several times. The reaction mixture was stirred at 20 C for12 hours under H2 (o psi), filtered and the filtrate was concentrated in vacuo. Theresidue was purified by column chromatography (Si02, PE: EtOAc = 1:0 to 100:4) to give the title compound (19.82 g, 43 % yield, 96.4 % purity on LCMS) as a brown oil. 1H NMR (CDC13): 6 7.01 (t, 1 H), 6.71 (d, 1 H), 6.i (d, 1 H), 3.57 (br 5, 2 H), 2.93 (t, 2H), 2.75 (t, 2 H) and 2.16-2.08 (m, 2 H).LCMS: m/z 134.2 (M+H) (ESI. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping