|
With cesium hydroxide; 4,5-bis(diphenylphos4,5-bis(diphenylphosphino)-9,9-dimethylxanthenephino)-9,9-dimethylxanthene;tris-(dibenzylideneacetone)dipalladium(0); In N,N-dimethyl-formamide; at 70℃; for 0.5h; |
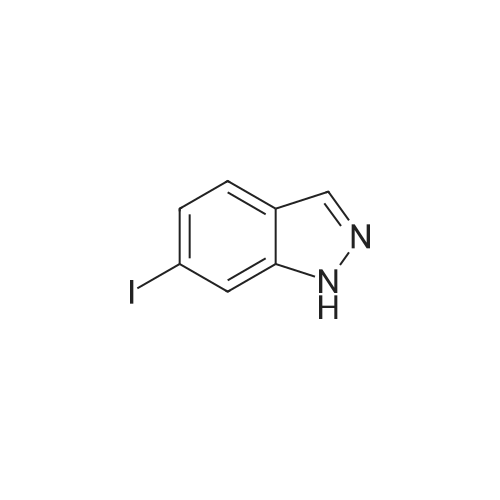
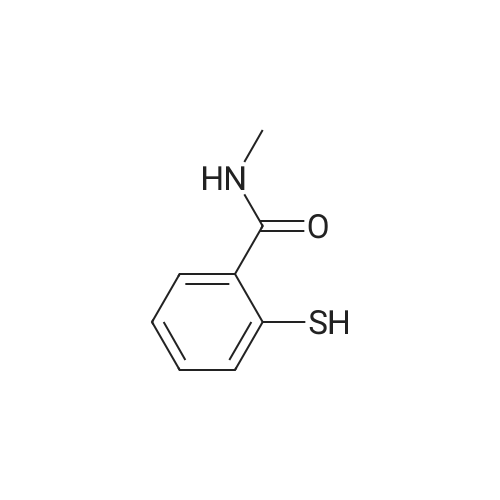
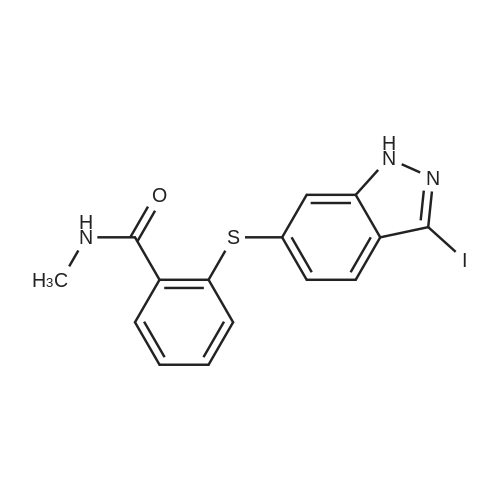
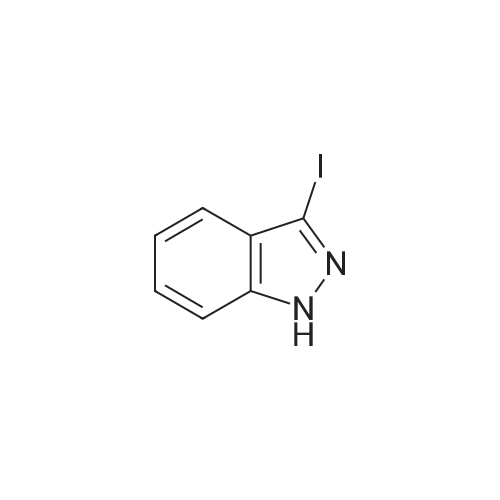
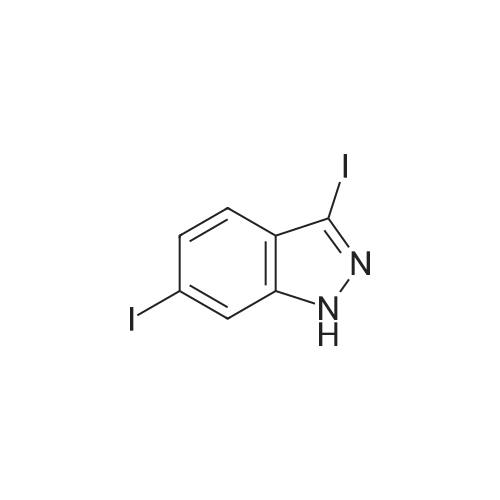
<strong>[319472-78-1]3,6-diiodoindazole</strong> (250.00 g), 2-mercapto-N-methylbenzamide (118.48 g), Pd2(dba)3 (9.28 g), Xantphos (11.73 g), DMF (2.5 L, 10 mL/g), followed by CsOH were added sequentially to a 5 L four-neck flask equipped with a mechanical stirrer and a temperature probe. The reaction mixture was then stirred. The dark mixture was degassed three times by alternately connecting to house vacuum and then nitrogen. The mixture was heated to 70 C over a period of 30 minutes and maintained at the same temperature for fours, at which time HPLC of the EPO <DP n="30"/>aliquot indicated that the <strong>[319472-78-1]3,6-diiodoindazole</strong> was less than 3%. After cooling, the mixture was poured into a mixture of 7.5 L of water, 1.25 L of toluene and 1.25 L of CH2CI2 in a 22 L extractor. The mixture was allowed to stir at ambient temperature overnight. A thick precipitate formed overnight. The mixture was filtered and the cake was sucked dry. The cake was further dried at 35 C under house vacuum for six hours to afford 216 g of the final product. The mother liquor was then extracted with 1.5 L of EtOAc. After partitioning, the aqueous layer was discarded. The organic layer was washed twice each with 2 L of water and concentrated. The residue was treated with 250 mL of CH2CI2 and stored overnight. A thick precipitate formed overnight. The mixture was filtered and the cake was sucked dry. The cake was dried at 35 0C under house vacuum overnight to afford 24.71 g of the final product. The combined yield was 241 g of the final product. The material showed satisfactory purity and was used in the next step without further purification. 1H NMR 300MHz, DMSO ppm: 13.53 (s, 1 H), 8.35 (q, J=4.7 Hz, 1 H), 7.56 (s, 1H), 7.51-7.40 (m, 2H), 7.36-7.23 (m, 3H), 7.13 (dd, J=8.5, 1.3 Hz, 1H), 7.06-7.01 (m, 1 H), 2.76 (d, J=4.7 Hz, 3H). |
|
With cesium hydroxide; 4,5-bis(diphenylphos4,5-bis(diphenylphosphino)-9,9-dimethylxanthenephino)-9,9-dimethylxanthene;tris-(dibenzylideneacetone)dipalladium(0); In N,N-dimethyl-formamide; at 20 - 70℃;Inert atmosphere; |
<strong>[319472-78-1]3,6-diiodoindazole</strong> (250.00 g), 2 -mercapto-N-methylbenzamid e (118.48 g), Pd 2(dba)3 (9.28 g), Xantphos (11.73 g), DMF (2.5 L, 10 mL/g), followed by CSOH were added sequentially to a 5 L four-neck flask equipped with a mechanical stirrer and a temperature probe. The reaction mixture was then stirred. The dark mixt ure was degassed three times by alternately connecting to house vacuum and then nitrogen. The mixture was heated to 70 C over a period of 30 minutes and maintained at the same temperature for fours, at which time HPLC of the aliquot indicated that the 3, 6-diiodoindazole was less than 3%. After cooling, the mixture was poured into a mixture of 7.5 L of water, 1.25 L of toluene and 1.25 L of CH2Cl2 in a 22 L extractor. The mixture was allowed to stir at ambient temperature overnight. A thick precipitate formed overnight. The mixture was filtered and the cake was sucked dry. The cake was further dried at 35 C under house vacuum for six hours to afford 216 g of the final productproduct. The mother liquor was then extracted with 1.5 L of EtOAc. After partition ing, the aqueous layer was discarded. The organic layer was washed twice each with 2 L of water and concentrated. The residue was treated with 250 mL of CH 2Cl2 and stored overnight. A thick precipitate formed overnight. The mixture was filtered and the cake was sucked dry. The cake was dried at 35 C under house vacuum overnight to afford 24.71 g of the final productproduct. The combined yield was 241 g of the final productproduct. The material showed satisfactory purity and was used in the next step without furth er purification. 1H NMR 300MHz, DMSO ppm: 13.53 (s, 1H), 8.35 (q, J=4.7 Hz, 1H), 7.56 (s, 1H), 7.51 - 7.40 (m, 2H), 7.36-7.23 (m, 3H), 7.13 (dd, J=8.5, 1.3 Hz, 1 H), 7.06 -7.01 (m, 1 H), 2.76 (d, J=4.7 Hz, 3H). |
|
With cesium hydroxide;tris-(dibenzylideneacetone)dipalladium(0); 4,5-bis(diphenylphos4,5-bis(diphenylphosphino)-9,9-dimethylxanthenephino)-9,9-dimethylxanthene; In N,N-dimethyl-formamide; at 70℃; for 4.5h;Product distribution / selectivity; |
Example 2 Preparation of 2-(3-Iodo-1H-indazol-6-ylsulfanyl)-N-methyl-benzamide <strong>[319472-78-1]3,6-diiodoindazole</strong> (250.00 g), 2-mercapto-N-methylbenzamide (118.48 g), Pd2(dba)3 (9.28 g), Xantphos (11.73 g), DMF (2.5 L, 10 mL/g), followed by CsOH were added sequentially to a 5 L four-neck flask equipped with a mechanical stirrer and a temperature probe. The reaction mixture was then stirred. The dark mixture was degassed three times by alternately connecting to house vacuum and then nitrogen. The mixture was heated to 70 C. over a period of 30 minutes and maintained at the same temperature for fours, at which time HPLC of the aliquot indicated that the <strong>[319472-78-1]3,6-diiodoindazole</strong> was less than 3%. After cooling, the mixture was poured into a mixture of 7.5 L of water, 1.25 L of toluene and 1.25 L of CH2Cl2 in a 22 L extractor. The mixture was allowed to stir at ambient temperature overnight. A thick precipitate formed overnight. The mixture was filtered and the cake was sucked dry. The cake was further dried at 35 C. under house vacuum for six hours to afford 216 g of the final productproduct. The mother liquor was then extracted with 1.5 L of EtOAc. After partitioning, the aqueous layer was discarded. The organic layer was washed twice each with 2 L of water and concentrated. The residue was treated with 250 mL of CH2Cl2 and stored overnight. A thick precipitate formed overnight. The mixture was filtered and the cake was sucked dry. The cake was dried at 35 C. under house vacuum overnight to afford 24.71 g of the final productproduct. The combined yield was 241 g of the final productproduct. The material showed satisfactory purity and was used in the next step without further purification. 1H NMR 300 MHz, DMSO ppm: 13.53 (s, 1H), 8.35 (q, J=4.7 Hz, 1H), 7.56 (s, 1H), 7.51-7.40 (m, 2H), 7.36-7.23 (m, 3H), 7.13 (dd, J=8.5, 1.3 Hz, 1H), 7.06-7.01 (m, 1H), 2.76 (d, J=4.7 Hz, 3H). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping