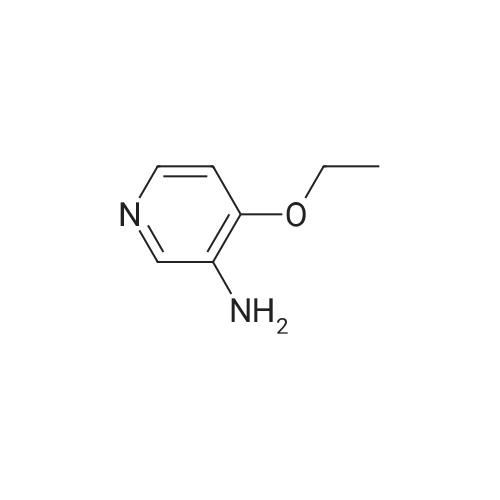
| 100% |
With hydrogen;palladium 10% on activated carbon; In ethanol; at 20℃; under 2585.81 Torr; for 6h; |
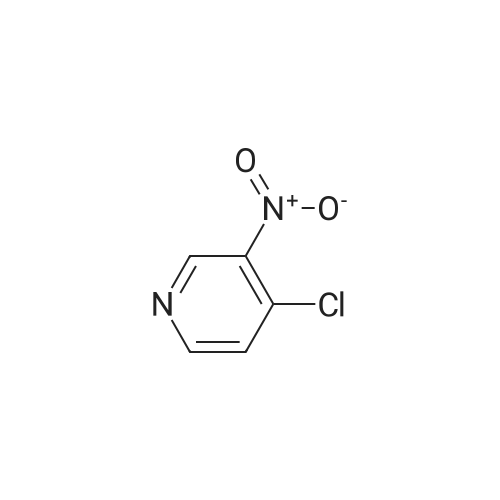
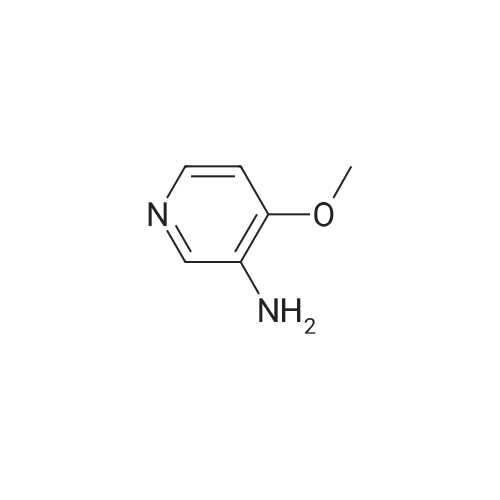
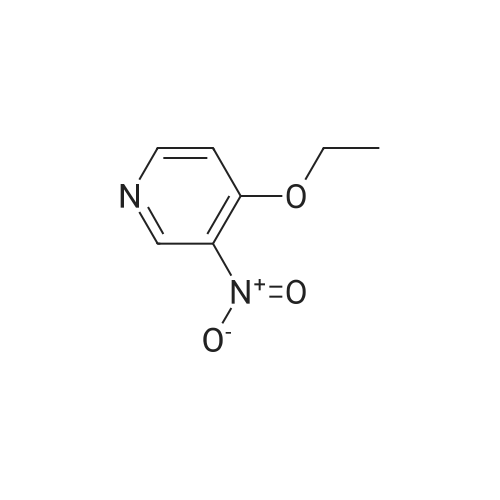
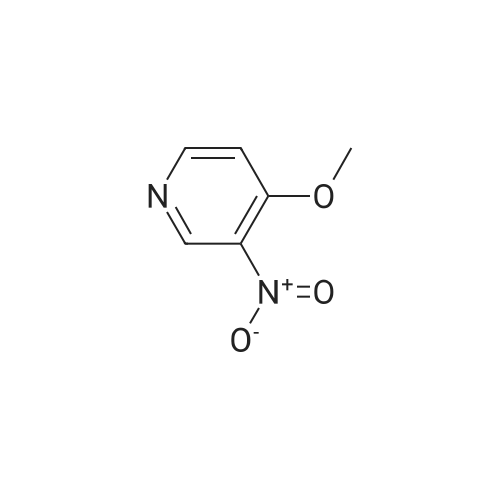
EXAMPLE 93; Synthesis of 4-Hydroxy-4-[3-(trifluoromethy)phenyl]piperidine-1-carboxylic acid (7-methoxy-thiazolo[5,4-b]pyridine-2-yl)-amide; Step 1: To a mixture of <strong>[31872-62-5]4-Methoxy-3-nitropyridine</strong> (5.0 g, 32.44 mmole) in ethanol (100 mL) was added 10 % palladium on carbon catalyst (200 mg). The resulting mixture was allowed to shake under a hydrogen atmosphere (50 psi) for 6 h. at room temperature. TLC (50% ethyl acetate/hexane) indicated complete consumption of starting material. Filtration through celite to remove the catalyst and concentration gave 3-Amino4 methoxypyridine (4.0 g, 32.44 mmol, 100% yield) as dark red oil. |
| 99% |
With hydrogen;palladium 10% on activated carbon; In ethanol; at 20℃; for 48h; |
Example 26Synthesis of 4-[2-(difluoromethyl)-1H-benzimidazol-1-yl]-N-(4-methoxy-3-pyridinyl)-6-(4-morpholinyl)-1,3,5-triazin-2-amineThe compound was synthesized according to Method A.A mixture of 0.475 g (3.08 mmol) of <strong>[31872-62-5]4-methoxy-3-nitropyridine</strong> (Org. Process Res. Dev. 2004, 8, 903-908) and 0.301 g (2.84 mmol) of 10% palladium on carbon in ethanol (30 mL) was stirred under an atmosphere of hydrogen for 48 hrs. The catalyst was removed by filtration through a pad of celite, and the solvent was removed to give 0.380 mg (99%) of 3-amino-4-methoxypyridine as a pink powder, which was used in the next step without further purification: 1H NMR (DMSO-d6) delta8.09 (dd, J=6.4, 1.2 Hz, 1H), 7.93 (d, J=1.2 Hz, 1H), 7.36 (d, J=6.4 Hz, 1H), 6.01 (br s, 2H), 4.06 (s, 3H).To a solution of 0.134 g (1.08 mmol) of 3-amino-4-methoxypyridine in THF (3 mL) was added 0.5 mL of butyllithium (2.5 M solution in hexanes), and the mixture was stirred for 15 min. A solution of 0.133 g (0.36 mmol) of 1-[4-chloro-6-(4-morpholinyl)-1,3,5-triazin-2-yl]-2-(difluoromethyl)-1H-benzimidazole in THF (6 mL) was added and the resulting mixture was stirred for 1 hr. After neutralization with acetic acid, the mixture was diluted with water and extracted with EtOAc. The organic layer was washed sequentially with water and aq. NH3, dried, and concentrated. Chromatography on alumina, eluting first with hexanes/EtOAc (1:1) and then with CH2Cl2/EtOAc (2:3) gave a white powder. Recrystallization from ethanol/CH2Cl2 gave 0.078 g (48% yield) of 4-[2-(difluoromethyl)-1H-benzimidazol-1-yl]-N-(4-methoxy-3-pyridinyl)-6-(4-morpholinyl)-1,3,5-triazin-2-amine: mp 161-163 C.; 1H NMR (DMSO-d6) delta9.43 (br s, 1H), 8.61-8.37 (m, 3H), 7.83-7.81 (m, 2H), 7.41 (br s, 2H), 7.20 (d, J=5.6 Hz, 1H), 3.89 (s, 3H), 3.81 (s, 4H), 3.71 (s, 4H); Anal. Calcd. for C21H20F2N8O2: C, 55.5; H, 4.4; N, 24.7. Found: C, 55.5; H, 4.4; N, 24.5%. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping