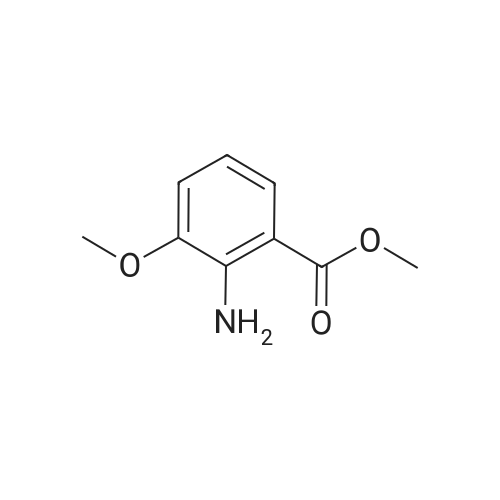
| 78.4% |
With sulfuric acid; at 90℃; for 48h; |
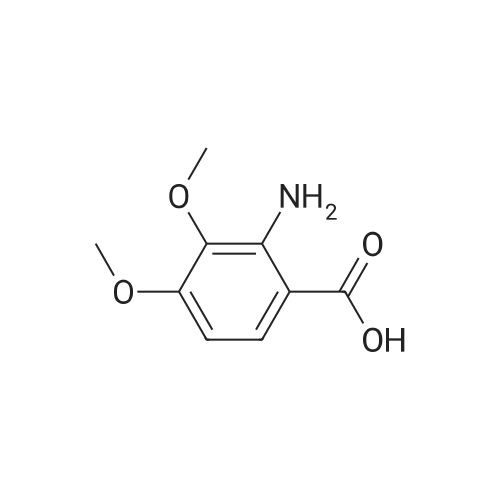
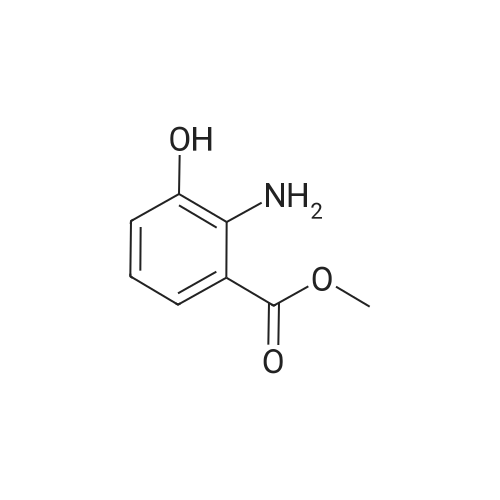
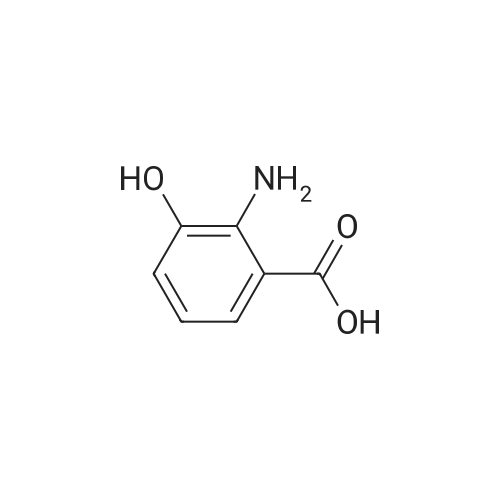
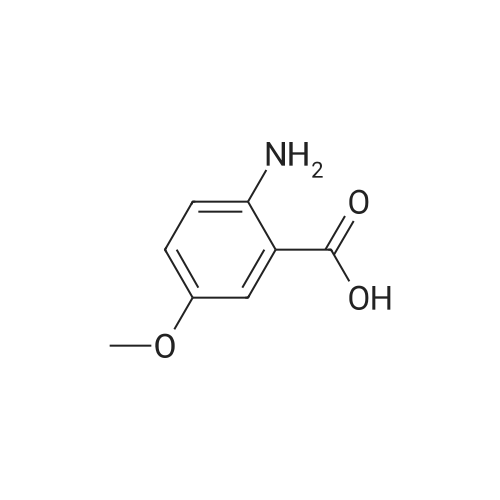
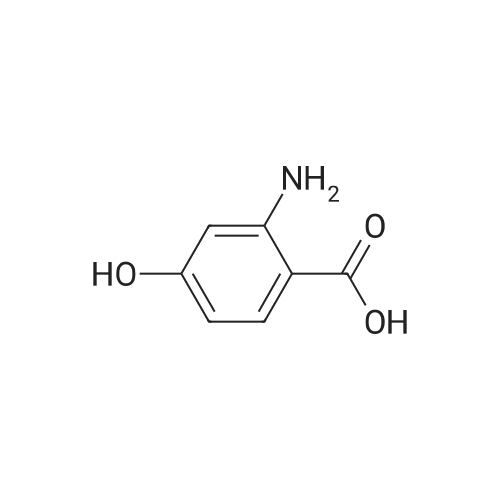
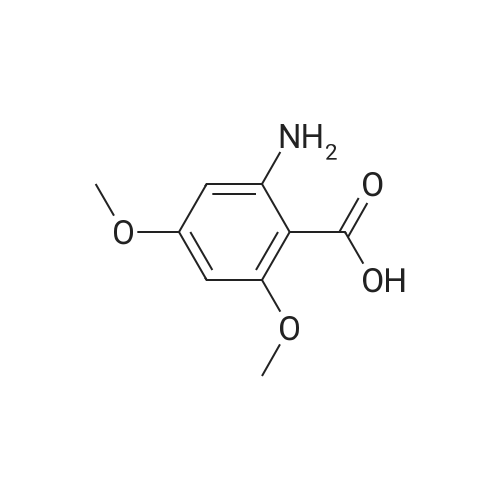
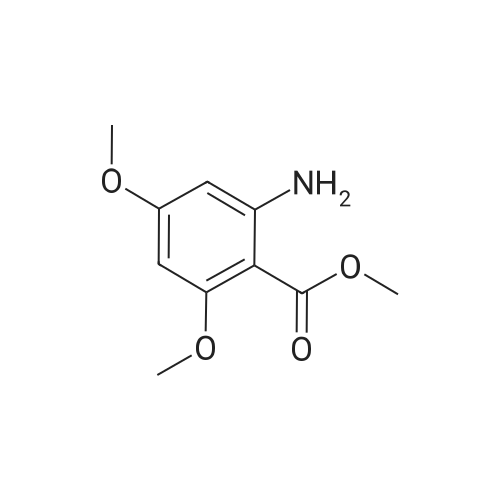
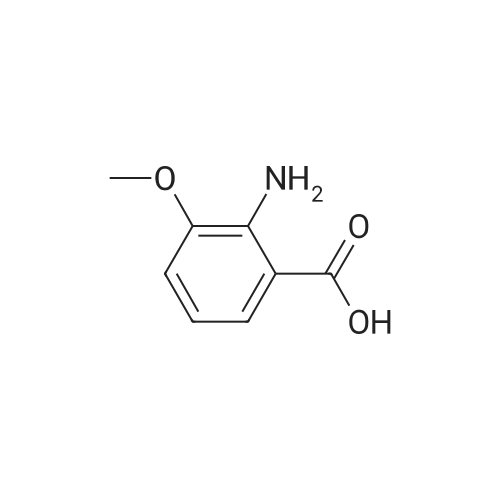
To a solution of 2-amino-3-methoxybenzoic acid (20 g, 119.64 mmol, 1 eq) in MeOH (250 mL) was added H2S04 (55.20 g, 551.56 mmol, 30 mL, 98% purity, 4.61 eq). The mixture was stirred at 90C for 48 h. The reaction mixture was concentrated under reduced pressure to remove MeOH. The residue was diluted with H20 (100 mL) and was added saturated aqueous NaHC03 until pH=8. The aqueous solution was extracted with EtOAc (50 mL x 3). The combined organic layers were washed with brine (75 mL x 2), dried over Na2S04, filtered and concentrated under reduced pressure to give methyl 2-amino-3-methoxybenzoate (17 g, 93.83 mmol, 78.4% yield) as brown oil. 1H NMR (CDCL, 400 MHz): d 7.48 (d, / = 7.2 Hz, 1H), 6.86 (d, / = 6.8 Hz, 1H), 6.58 (t, J = 8.0 Hz, 1H), 6.01 (br s, 2H), 3.88 (s, 6H) ppm. To a solution of methyl 2-amino-3-methoxybenzoate (16.5 g, 91.07 mmol, 1 eq) in DMF (200 mL) was added NCS (12.53 g, 93.80 mmol, 1.03 eq) at 25C. The resulting mixture was stirred and heated at 50C for 2 h. The reaction mixture was quenched by addition ice-water (500 mL) at 0C, and then extracted with EtOAc (100 mL x 3). The combined organic layers were washed with brine (300 mL x 3), dried over Na2S04, filtered and concentrated under reduced pressure to give methyl 2-amino-5-chloro-3- methoxybenzoate (19 g, 88.11 mmol, 96.8% yield) as brown oil, which was used into the next step without further purification. 'H NMR (CDCL, 400 MHz): d 7.46 (d, J = 2.0 Hz, 1H), 6.79 (d, J = 2.4 Hz, 1H), 6.01 (br s, 2H), 3.87 (s, 6H) ppm. To a solution of methyl 2-amino-5-chloro-3-methoxybenzoate (19 g, 88.11 mmol, 1 eq) in CH3CN (300 mL) was added CuBr2 (40 g, 179.09 mmol, 8.39 mL, 2.03 eq) resulting in a dark color. The mixture was stirred for 20 min at 25C, and t-BuONO (16.36 g, 158.60 mmol, 18.86 mL, 1.8 eq) was added dropwise over 10 min. The reaction mixture was stirred for additional 30 min, and then heated at 60C for 12 h. The reaction mixture was concentrated in vacuo, and water (300 mL) and EtOAc (100 mL) were added. The resulting mixture was stirred at 25C for 30 min. The organic phase became brown, and the aqueous was green with insoluble materials. The whole mixture was filtered through Celite and washed with EtOAc (100 mL x 3). The organic layer was separated, washed with brine (100 mL x 3), dried over Na2S04, filtered and concentrated under reduced pressure to give a residue. The residue was purified by column chromatography (Si02, petroleum ether/ethyl acetate = 10/1 to 3/1) to give methyl 2-bromo-5-chloro-3-methoxybenzoate (16 g, 57.24 mmol, 65.0% yield) as a white solid. 1H NMR (CDCl , 400 MHz): d 7.28 (d, / = 2.4 Hz, 1H), 6.98 (d, J = 2.4 Hz, 1H), 3.94 (s, 3H), 3.93 (s, 3H) ppm. To a solution of methyl 2- bromo-5-chloro-3-methoxybenzoate (10 g, 35.78 mmol, 1 eq) in DCM (300 mL) was slowly added BBr3 (26.89 g, 107.33 mmol, 10.34 mL, 3 eq) at -78C under N2. To the reaction mixture was slowly added MeOH (100 mL), and the resulting mixture was stirred at 20C for 30 min. It was mixed with ice-water 500 mL at 0C, and the organic phase was separated. The aqueous was extracted with DCM (100 mL x 3). The combined organic layers were washed with brine (200 mL x 2), dried over Na2S04, filtered and concentrated under reduced pressure to give a residue. The residue was purified by column chromatography (Si02, petroleum ether/ethyl acetate = 10/1 to 0/1) to give methyl 2-bromo-5-chloro-3 -hydroxy-benzoate (4 g, 15.07 mmol, 42.1% yield) as a yellow solid. 1H NMR (CDCl , 400 MHz): d 7.43 (d, / = 2.4 Hz, 1H), 7.20 (d, / = 2.4 Hz, 1H), 6.09 (s, 1H), 3.95 (s, 3H) ppm. To a solution of methyl 2-bromo-5-chloro-3- hydroxybenzoate (0.9 g, 3.39 mmol, 1 eq) in DMF (15 mL) and H20 (1.5 mL) were added sodium 2-chloro-2,2-difluoro-acetate (1.81 g, 11.86 mmol, 3.5 eq) and K2C03 (937.03 mg, 6.78 mmol, 2 eq) at 20C. The reaction was stirred under argon at l00C for 5 h. The reaction mixture was quenched by addition H20 (30 mL) at 20C, and then the aqueous was extracted with EtOAc (15 mL x 3). The combined organic layers were washed with brine (20 mL x 3), dried over Na2S04, filtered and concentrated under reduced pressure to give methyl 2-bromo-5-chloro-3-(difluoro methoxy)benzoate (750 mg, 2.38 mmol, 70.1% yield) as a yellow solid. 1H NMR (CDCl3, 400 MHz): d 7.60 (d, / = 2.4 Hz, 1H), 7.37 (d, / = 2.4 Hz, 1H), 6.56 (t, / = 72.8 Hz, 1H), 3.96 (s, 3H) ppm. A mixture of methyl 2-bromo-5-chloro-3-(difluoromethoxy)benzoate (0.7 g, 2.22 mmol, 1 eq), Pin2B2 (2.82 g, 11.09 mmol, 5 eq), KOAc (544.37 mg, 5.55 mmol, 2.5 eq), and Pd(PPh3)2Cl2 (155.73 mg, 221.87 umol, 0.1 eq) in l,4-dioxane (20 mL) was degassed and purged with N2 for 3 times, and then the mixture was stirred at l20C for 5 h under N2 atmosphere. The reaction was cooled and filtered. The filtrate was concentrated under reduced pressure to give a residue. The residue was purified by column chromatography (Si02, petroleum ether/ethyl acetate = 30/1 to 5/1) to give methyl 5-chloro-3- (difluoromethoxy)-2-(4,4,5,5-tetram... |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping