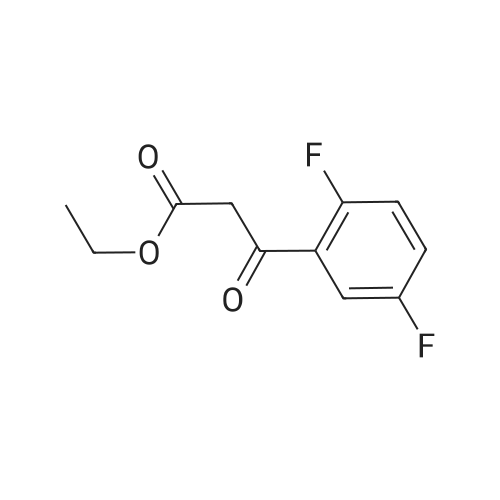
| 70% |
|
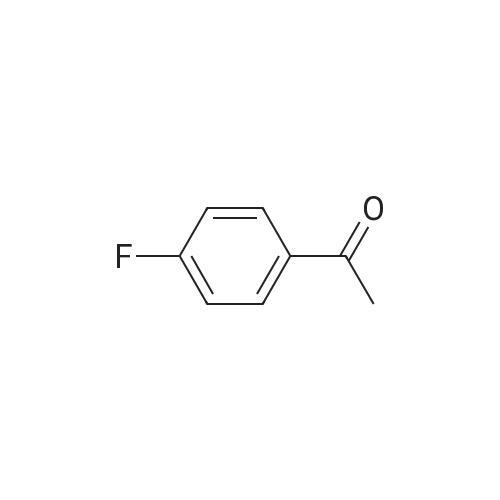
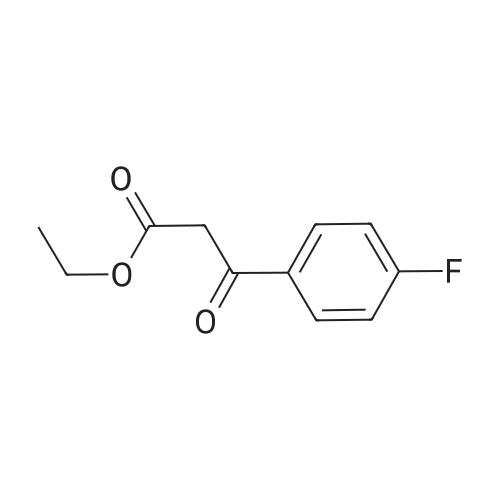
To a solution of sodium ethoxide (351 mL, 21% in ethanol, 1629 mmol) was added 1-(4-fluorophenyl) ethanone (150 g, 1086 mmol) in ethanol (100 mL) at 0 C. under a nitrogen atmosphere and the resulting reaction mixture was stirred at RT for 10 min Diethyl oxalate (156 mL, 1140 mmol) in ethanol (100 mL) was added and reaction was allowed to stir at RT for 12 h. Reaction mixture was cooled to 0 C. and acidified with 1.5 N HCl and the solid was filtered and the filtrate was diluted with water and extracted with DCM (3*750 mL). The combined organic layer was washed with brine, dried over Na2SO4, filtered and concentrated to afford Intermediate 1A (180 g, 70%) which was taken to next step without further purification. MS(ES): m/z=237 [M-H]+; 1H NMR (300 MHz, CDCl3) delta ppm 15.2 (bs, 1H), 8.00-8.09 (m, 2H), 7.15-7.25 (m, 2H), 7.05 (s, 1H), 4.42 (q, J=7.15 Hz, 2H), 1.43 (t, J=7.15 Hz, 3H). |
| 70% |
|
To a solution of sodium ethoxide (351 mL, 21% in ethanol, 1629 mmol) was added l-(4-fluorophenyl) ethanone (150 g, 1086 mmol) in ethanol (100 mL) at 0 C under a nitrogen atmosphere and stirred at RT for 10 min. Diethyl oxalate (156 mL, 1140 mmol) in ethanol (100 mL) was added and reaction was allowed to stir at RT for 12 h. The reaction mixture was cooled to 0 C and acidified with 1.5 N HCl and the solid was filtered and the filtrate was diluted with water and extracted with DCM (3 x 750 mL). The combined organic layer was washed with brine, dried over Na2S04, filtered and concentrated to afford Intermediate 1A (180 g, 70%), which was taken to next step without further purification. MS(ES): m/z = 237 [M-H]+; 1H NMR (300 MHz, CDC13) delta ppm 15.2 (bs, 1H), 8.00 - 8.09 (m, 2H), 7.15 - 7.25 (m, 2H), 7.05 (s, 1H), 4.42 (q, J = 7.15 Hz, 2H), 1.43 (t, J= 7.15 Hz, 3H) |
| 54% |
|
To a solution of diisopropyl amine (6.2mL, 44mmol) in THF (44mL) at 0C was added n-BuLi (16.2mL, 40.5mmol). The cloudy yellow solution was stirred at 0C for 30 min., then cooled to -78C. 4?-fluoroacetophenone (3.2mL, 26 mmol) was added slowly along the sides of the flask and was stirred for 15 min. Diethyl oxalate (7.9mL, 58 mmol) was added and the reaction stirred at -78C for 1 hour. The mixture was warmed to room temperature and stirred for 20 min and the reaction was quenched by the addition of 1M HCl. The organic solvent was removed by rotary evaporation. The aqueous phase was extracted with EtOAc (3× 75mL) and the combined organic layers were washed with 1M HCl (25mL), saturated aqueuos NaHCO3 (25mL), and brine (25mL). The organic phase was dried over Na2SO4, filtered, and concentrated. The crude material was purified by flash column chromatography and recrystallized from EtOH to obtain 2b (3.38 g, 54% yield) as a yellow solid. 1HNMR (500 MHz, CDCl3) delta 15.83 - 15.03 (m, 1H), 8.42 - 8.09 (m, 2H), 7.50 (s, 1H), 7.42 (t, J= 8.5Hz, 2H), 4.64 (q, J= 7.1Hz, 2H), 1.65(t, J= 7.2Hz, 3H). 13C NMR (125 MHz, CDCl3) delta 189.86, 169.33, 166.36 (d, J= 256.5Hz), 162.27, 131.50 (d, J= 2.5Hz), 130.72 (d,J= 9.5Hz) , 116.3 (d, J= 22.0Hz) , 97.96, 62.83, 14.25. |
| 54% |
|
To a solution of diisopropyl amine (6.2 mL, 44 mmol) in THF (44 mL) at 0 C was added n-BuLi (16.2 mL, 40.5 mmol). The cloudy yellow solution was stirred at 0 C for 30 min., then cooled to -78 C. 4'-fluoroacetophenone (3.2 mL, 26 mmol) was added slowly along the sides of the flask and was stirred for 15 min. Diethyl oxalate (7.9 mL, 58 mmol) was added and the reaction stirred at -78 C for 1 hour. The mixture was warmed to room temperature and stirred for 20 min and the reaction was quenched by the addition of 1M HC1. The organic solvent was removed by rotary evaporation. The aqueous phase was extracted with EtOAc (3 x 75 mL) and the combined organic layers were washed with 1M HC1 (25 mL), saturated aqueuos NaHC03 (25 mL), and brine (25 mL). The organic phase was dried over Na2S04, filtered, and concentrated. The crude material was purified by flash column chromatography and recrystallized from EtOH to obtain 2b (3.38 g, 54 % yield) as a yellow solid. NMR (500 MHz, CDCl3) d 15.83 - 15.03 (m, 1H), 8.42 - 8.09 (m, 2H), 7.50 (s, 1H), 7.42 (t, J = 8.5 Hz, 2H), 4.64 (q, J = 7.1 Hz, 2H), 1.65 (t, J = 7.2 Hz, 3H). 13C NMR (125 MHz, CDCl3) d 189.86, 169.33, 166.36 (d, J = 256.5 Hz), 162.27, 131.50 (d, J = 2.5 Hz), 130.72 (d, J = 9.5 Hz)*, 116.3 (d, J = 22.0 Hz)*, 97.96, 62.83, 14.25. |
|
|
Example 54 Ethyl 4-(4-fluorophenyl)-2,4-dioxobutanoate To a solution of sodium metal (1.03 g, 45.0 mmol) in ethanol (100 mL) were slowly added drops of 1-(4-fluorophenyl)ethanone (4.50 mL, 36.9 mmol) at 0 C. The solution was stirred for 30 min, and then slowly added drops of diethyl oxalate (5.50 mL, 40.5 mmol) at the same temperature. Following stirring overnight at room temperature, the progression of the reaction was monitored by TLC (Hexane:EtOAc=4:1). When the reaction was completed, the reaction mixture was concentrated in vacuo. To the concentrate, 6M HCl was added dropwise at 0 C., followed by extraction with dichloromethane and water. The organic layer thus formed was dried over anhydrous magnesium sulfate, filtered, and concentrated to afford the title compound without further purification (9.07 g, quant., yellow solid). 1H NMR (300 MHz, CDCl3) delta 8.09-8.04 (m, 2H), 7.24-7.20 (m, 2H), 7.07 (s, 1H), 4.44 (q, J=7.2 Hz, 2H), 1.45 (t, J=7.2 Hz, 3H) |
|
|
To a stirred solution of NaH (60%) (15.0 g, 361mmol), in toluene (400mL) at 0 C, was added 4-fluoro acetophenone (25.0 g, 181mmol) drop wise at 0 C. The reaction mixture was then stirred at 0 C for 30 minutes. Diethyl oxalate (37mL, 271mmol) was added drop wise at 0 C. The reaction mixture was stirred at 25 C for 2h. The reaction mixture was diluted with water (lOOOmL) and extracted in ethyl acetate (250mL x 3). The organic layer was washed with brine (250mL), dried over anhydrous sodium sulphate and distilled off to obtain crude ethyl 4-(4-fluorophenyl)-2,4- dioxobutanoate (44.0 g) as a liquid. This was carry forward to next step without further purification. (238.96 [M+H]). |
|
With sodium ethanolate; In ethanol; at 70℃; for 5h; |
General procedure: 0.1 mol (1 eq) of substituted acetophenone and 0.2 mol (29.2 g, 2 eq) of diethyl oxalate were weighed into a 250 ml three-Add 100ml absolute absolute ethanol,Slowly drop the newly prepared ethanol solution of sodium ethoxide,Plus,The temperature was raised to 70 C for 5 hours,TLC detection,No raw materials to replace acetophenone,Reaction finished,The system is reddish brown.Stop heating,The solvent was distilled off under reduced pressure,Dark red brown sticky material,With about 100 ml of water transferred to 200 ml of ice water,With concentrated hydrochloric acid 8ml adjusted to pH 1,Stirring for 30min, filtering,The filter cake was washed with ice ethanol.; The substituted acetophenones are p-fluoroacetophenone, p-chloroacetophenone, p-bromoacetophenone, p-methylacetophenone and p-trifluoro(2a-2e) are ethyl 4- (4-fluorophenyl) -2,4-dioxobutyrate, 4- (4- (4-fluorophenyl) Chlorophenyl) -2,4-dioxobutyrate, ethyl 4- (4-bromophenyl) -2,4-dioxobutyrate, 4- (4-methylphenyl) 2,4-dioxobutyrate, ethyl 4- (4-trifluoromethylphenyl) -2,4-dioxobutyrate, as follows: |
|
With sodium hydride; In toluene; mineral oil; at 50℃; for 1.5h;Reflux; |
General procedure: To a stirred solution of appropriate methyl ketone (0.1 mol) in dry toluene (200 mL)was added a suspension of NaH in mineral oil (60%; 0.2 mol) in portions and the mixture was warmed to50 C. At this temperature a solution of diethyl oxalate (0.15 mol) in dry toluene (60 mL) was addeddropwise under stirring. The reaction mixture was refluxed for 1.5 h. Upon cooling to room temperatureacetic acid (0.25 mol) was added dropwise. The reaction mixture was washed with water (200 mL), organicphase was dried over MgSO4 and evaporated to dryness. The obtained crude diketone was dissolved inethanol (250 mL), hydrazine dihydrochloride (0.11 mol) was added and the mixture was refluxed for 3 h.After evaporation of solvent the residue was treated with water (200 mL) and kept under ice-cooling for 1 h.Crystals were filtered off and dried in air to afford the corresponding pyrazole. In some cases thus obtainedsubstance was purified by recrystallization from aqueous ethanol. |
|
|
Sodium metal (60%, 15.06 g, 376 mmol) is added to dry ethanol (300 ml_), then 1-(4-fluorophenyl)- ethanone (40.0 g, 289 mmol) in dry THF is added at 0 C and stirred for 10 min. at this temperature. Diethyl oxalate (50.78 g, 347 mmol) is added and stirred for 16 h at ambient temperature. 2 N HCI is added. The formed solid is collected by filtration and dried. |
|
With potassium tert-butylate; In N,N-dimethyl-formamide; at 0 - 45℃; for 1.75h; |
In a three-necked flask acetophenone (5g, 41.61 mmol) anddiethyl oxalate (6.76 mL, 50 mmol) were dissolved in anhydrousDMF 65 mL at 0 C and t-BuOK (2g, 83.25 mmol) was slowly added,the mixture was stirred at 0 C for 15 min and 30 min more at roomtemperature before heating at 45 C for 1h. Reaction was pouredinto water-acetic acid and product extracted with ethyl acetate.Organic layers were combined, washed with water and brine, driedover magnesium sulphate, filtered and concentrated to yield thetitle compound. MS (ESI) m/z (%): 237.2 [M H] |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping